- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Manganese Cytotoxicity Assay on Hippocampal Neuronal Cell Culture
Published: Vol 5, Iss 1, Jan 5, 2015 DOI: 10.21769/BioProtoc.1368 Views: 9684
Reviewed by: Soyun KimHong-guang XiaKae-Jiun Chang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Time-Lapse Super-Resolution Imaging and Optical Manipulation of Growth Cones in Elongating Axons and Migrating Neurons
Masato Sawada [...] Kazunobu Sawamoto
Mar 20, 2025 2139 Views

Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
Hanki Kim [...] Jun Young Choi
Jun 20, 2025 1426 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2637 Views
Abstract
Compared to an in vivo experiment, neuronal cell cultures are immediately accessible to observation and manipulation. In this protocol, we describe a technique to evaluate the cytotoxicity of a metal, manganese (Mn2+), on hippocampal neuronal cell cultures. Interestingly, this protocol is easily adaptable to any type of primary culture (e.g., cortical neurons) and any type of toxic compound (e.g., chemical product).
This protocol is similar to "Neuron-enriched Cultures (Method 2)" protocol (Gao, 2011).
Materials and Reagents
- Pregnant mouse (embryonic day: E18.5)
- Borax (Sigma-Aldrich, catalog number: 71997 )
- Boric acid (Sigma-Aldrich, catalog number: B6768 )
- 50x B27 supplement (Life Technologies, InvitrogenTM, catalog number: 17504-044 )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418 )
- Dulbeccos modified Eagle medium (DMEM) (Life Technologies, InvitrogenTM, catalog number: 31966-021)
- Fetal bovine serum (FBS) (Life Technologies, InvitrogenTM, catalog number: 16000-044 )
- 100x glutamax (Life Technologies, InvitrogenTM, catalog number: 35050-038)
- 10x HBSS (Life Technologies, InvitrogenTM, catalog number: 14185-052 )
- 1 M MnCl2 (Sigma-Aldrich, catalog number: M1787 )
- Neurobasal medium (Life Technologies, InvitrogenTM, catalog number: 21103-049 )
- Penicillin-streptomycin (PS) (10,000 U/ml) (Life Technologies, InvitrogenTM, catalog number: 15140-122 )
- Dulbecco’s phosphate buffer saline (DPBS) with Ca2+ and Mg2+ (Sigma-Aldrich, catalog number: D8662 )
- Poly-L-Lysine hydrobromide (Sigma-Aldrich, catalog number: P2636 )
- Sterile water
- Thiazolyl blue tetrazolium bromide (MTT) (Sigma-Aldrich, catalog number: M2128 )
- 10x trypsine (Life Technologies, InvitrogenTM, catalog number: 15090-046 )
- Borate buffer (see Recipes)
- 1 mg/ml poly-L-lysine (see Recipes)
- First culture medium (see Recipes)
- 1x HBSS (see Recipes)
- Second culture medium (see Recipes)
- MnCl2 (0, 20, 50, 100 and 150 µM) (see Recipes)
- 0.5 mg/ml MTT (see Recipes)
Equipment
- One sterile curved plier and one sterile sharp plier (Figure 1)
- One bag of sterile plastic Petri dishes (100 mm diameter)
- 24 wells plate (sterile)
- 96 wells plate (non sterile)
- Syringe filter
- Pipette P1000 with a P1000 cone and a P200 cone (sterile)
- Neubauer or Malassez cell counting chamber
- Eppendorf tubes
- Cell scraper
- Tissue culture hood
- Optic microscope inside the hood
- Water bath at 37 °C
- Incubator at 37 °C (humidified atmosphere with 5% CO2)
- Microplate reader (Pherastar plus) (BMG LABTECH)
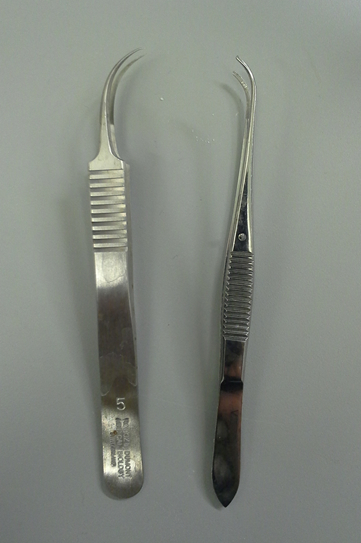
Figure 1. Pliers for the hippocampi dissection
Procedure
Note: All steps are performed under a cell culture hood, using aseptic techniques.
- Well plate preparation (Day 1-2)
Day 1- Place 300 µl of poly-L-lysine 1 mg/ml in all wells of the 24 well plate. Poly-L-lysine is an attachment factor, which improves cell adherence.
- Incubate over night at room temperature.
- Remove the poly-L-lysine.
- Rinse 2 times (quickly), then rinse for 1 h and finish with 2 rinses (quickly) with sterile water.
- Add 1.5 ml of the first culture medium per well.
- Place the plate in the incubator until step B9.
- Place 300 µl of poly-L-lysine 1 mg/ml in all wells of the 24 well plate. Poly-L-lysine is an attachment factor, which improves cell adherence.
- Hippocampal neurons preparation (Day 2)
- Remove hippocampi from the embryo brain in 1x HBSS using the pliers and the microscope, Figure 1 [see Figure 1A-F in the article of Fuller and Dailey (Fuller and Dailey, 2007)].
- Place hippocampi in a tube with 4.5 ml of 1x HBSS.
- Add 500 µl of 10x trypsin.
- Incubate in a water bath at 37 °C (98.6 °F) for 15 min.
- Rinse 3 times with 1x HBSS leaving the tissue at the bottom of the tube.
- Add 500 µl of the first culture medium.
- Using a P1000 pipette with a P1000 cone and a P200 cone, perform the mechanical dissociation (Figure 2) by sucking and spitting several times the cone content with the pipette. During this step, be careful not to create air bubbles in the medium (air bubbles are toxic for neurons).
- Wait a few minutes until the aggregate settles to the bottom of the tube.
- Transfer supernatant containing dispersed cells to a sterile Eppendorf tube. Leave ~25 µl of culture medium containing aggregate.
- With a Neubauer or Malassez cell counting chamber, count the number of cells and adjust to a number of 2.2 x 105 cells per well. Usually, there are around 1 x 106 hippocampal cells per embryo, so one embryo can be used to fill 5 wells approximately.
- Place in the incubator for 2 h.
- Replace the first culture medium by the second culture medium (1.5 ml per well).
- Place in the incubator for 2 days (until part C).
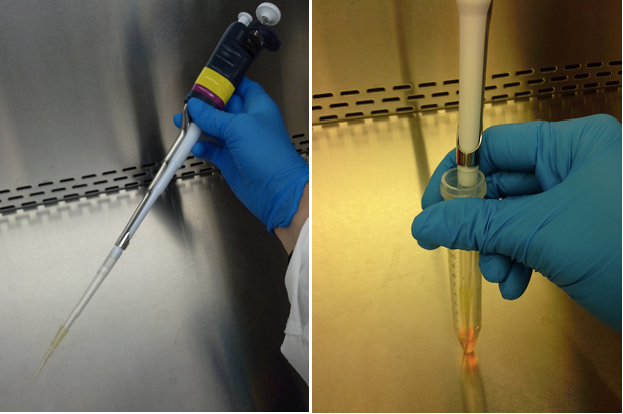
Figure 2. Mechanical dissociation using a P1000 pipette with a P1000 cone and a P200 cone
- Remove hippocampi from the embryo brain in 1x HBSS using the pliers and the microscope, Figure 1 [see Figure 1A-F in the article of Fuller and Dailey (Fuller and Dailey, 2007)].
- Addition of MnCl2 in the medium culture (Day 4)
In this protocol, 5 dilutions of MnCl2 are studied: 0, 20, 50, 100 and 150 µM.
Replace the culture medium by the Mn solutions at different concentrations and place the plate in the incubator for 24 h. - MTT assay (Day 5)
MTT assay is a fast method for the enumeration of living cells. The tetrazolium salt (yellow) is reduced into formazan by the mitochondrial succinate dehydrogenase of living cells. This reaction creates a purple precipitate.- Prepare fresh MTT solutions 5 mg/ml.
- Remove the culture medium and add 500 µl of MTT 0.5 mg/ml diluted in the second medium culture.
- Incubate the plate for 2 h at 37 °C.
- Induce cell lysis by replacing MTT solution by 500 µl of pure DMSO. Cells will liberate the purple precipitate formed by mitochondria of living cells.
- Using a P1000 pipette, help cell lysis by sucking and spitting several times with the pipette.
- Detach the last cells still attached to the plate with a cell scraper.
- Transfer the lysate into an Eppendorf tube.
- Take 200 µl of each lysate and place them in a 96 well plate.
- Fill one well with 200 µl of pure DMSO for reference. The optic density of this reference-which contains 0 cells-will be subtracted from the other optic densities.
- Using the spectrophotometer, read the optic density of each well filled with the lysate using a wavelength = 519 nm. An absorbance inferior to 1 is necessary to respect the law of Beer Lambert. If the absorbance is above 1, the lysate must be diluted in pure DMSO. The typical absorbance for a culture of 2.2 x 105 neurons at Day 5 is 0.93 ± 0.09. The well without Mn is considered to contain 100% of living cells. You can then compute the fraction of living cells in the other wells from the ratio between the optic density in the wells and the optic density in the well without Mn.
- Prepare fresh MTT solutions 5 mg/ml.
Recipes
- Borate buffer
Mix the boric acid 3.1 g/ml with borax 4.75 g/ml in distilled water
For a final volume of 50 ml of distilled water, weigh 155 g of boric acid and 237.5 g of borax Adjust the pH to 8.5 with 1 M NaOH
Sterilized the solution by filtration - 1 mg/ml poly-L-lysine
- Prepare a stock solution of poly-L-lysine 10 mg/ml diluted in borate buffer.
- For a final volume of 10 ml, weigh 100 mg of poly-L-lysine in 10 ml of borate buffer. Place the stock solution at -20 °C.
- Use the stock solution to make a final solution of poly-L-lysine at 1 mg/ml diluted in borate buffer. For a final volume of 10 ml, pipette 1 ml of stock solution + 9 ml of borate buffer.
- Prepare a stock solution of poly-L-lysine 10 mg/ml diluted in borate buffer.
- First culture medium
This first culture medium is composed of 10% FBS, 1% PS diluted in DMEM.
For a final volume of 40 ml, mix 4 ml of FBS, 0.4 ml of PS and 35.6 ml of DMEM. - 1x HBSS
10x HBSS is diluted in sterile water.
For a final volume of 50 ml, mix 5 ml of HBSS 10x with 45 ml of sterile water. - Second culture medium
The second culture medium is composed of glutamax 1x and B27 d=1/50 diluted in Neurobasal. For a final volume of 40 ml, mix 0.4 ml of glutamax, 0.8 ml of B27 and 38.8 ml of Neurobasal. - MnCl2 (0, 20, 50, 100 and 150 µM)
- Prepare a solution of MnCl2 at 1 mM in distilled water (from the solution at 1 M).
- For a final volume of 10 ml, mix 10 µl of MnCl2 1 M and 10 ml of distilled water.
- Then, prepare the different concentrations of MnCl2 in the second culture medium.
- The final volume of each well is 1.5 ml. Calculate the number of wells that you want to use for each concentration. For example, if you use 3 wells per concentration, you need 4.5 ml of MnCl2 solution (3 x 1.5 ml). The solution preparation is described in Table 1 for a final volume of 4.5 ml.
Table 1. Preparation of MnCl2 solutions at different concentrations. This example is given for 3 wells per concentration and 4 Mn concentrations.Concentration of MnCl2 (µM) Volume of MnCl2 1 mM (µl) Second culture medium (ml) 0 0 4.5 20 90 4.41 50 225 4.28 100 450 4.05 150 672 3.83
- Prepare a solution of MnCl2 at 1 mM in distilled water (from the solution at 1 M).
- 0.5 mg/ml MTT
- First, prepare a MTT solution at 5 mg/ml diluted in PBS with Ca2+ and Mg2+. For 1.2 ml of PBS, weigh 6 mg of MTT. Protect the solution from light.
- Second, prepare a MTT solution at 0.5 mg/ml in the second culture medium. For a final volume of 12 ml of culture medium, add 1.2 ml of MTT at 5 mg/ml.
- First, prepare a MTT solution at 5 mg/ml diluted in PBS with Ca2+ and Mg2+. For 1.2 ml of PBS, weigh 6 mg of MTT. Protect the solution from light.
Acknowledgments
AD received a stipend from the Région Rhône-Alpes-Cluster HVN.
References
- Daoust, A., Saoudi, Y., Brocard, J., Collomb, N., Batandier, C., Bisbal, M., Salome, M., Andrieux, A., Bohic, S. and Barbier, E. L. (2014). Impact of manganese on primary hippocampal neurons from rodents. Hippocampus 24(5): 598-610.
- Fuller, L. and Dailey, M. E. (2007). Preparation of rodent hippocampal slice cultures. CSH Protoc 2007: pdb prot4848.
- Gao, H. M. (2011). Neuron-enriched cultures (method 2). Bio-protocol Bio101: e150.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Daoust, A., Saoudi, Y., Brocard, J., Collomb, N., Batandier, C., Bisbal, M., Salomé, M., Andrieux, A., Bohic, S. and Barbier, E. L. (2015). Manganese Cytotoxicity Assay on Hippocampal Neuronal Cell Culture. Bio-protocol 5(1): e1368. DOI: 10.21769/BioProtoc.1368.
Category
Neuroscience > Cellular mechanisms > Cell isolation and culture
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










