- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24Gag
Published: Vol 4, Iss 24, Dec 20, 2014 DOI: 10.21769/BioProtoc.1366 Views: 11794
Reviewed by: Yu ChenAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

General Maintenance and Reactivation of iSLK Cell Lines
Ariana C. Calderón-Zavala [...] Ekaterina E. Heldwein
Jun 5, 2025 1893 Views

Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs
Jade Jansen [...] Neeltje A. Kootstra
Jul 20, 2025 2460 Views

Assembly and Mutagenesis of Human Coronavirus OC43 Genomes in Yeast via Transformation-Associated Recombination
Brett A. Duguay and Craig McCormick
Aug 20, 2025 3038 Views
Abstract
The fluorescence-linked antigen quantification (FLAQ) assay allows a fast quantification of HIV-1 p24Gag antigen. Viral supernatant are lysed and incubated with polystyrene microspheres coated with polyclonal antibodies against HIV-1 p24Gag and detector antibodies conjugated to fluorochromes (Figure 1). After washes, the fluorescence of microspheres is measured by flow cytometry and reflects the abundance of the antigen in the lysate. The speed, simplicity, and wide dynamic range of the FLAQ assay are optimum for many applications performed in HIV-1 research laboratories.
Keywords: Viral ProductionMaterials and Reagents
- Sphero Protein A Polystyrene Particles (6-8 µm) (Spherotech, catalog number: PAP-60-5 )
- Human anti-p24Gag HIV-1 IIIB polyclonal antibodies (ImmunoDX, catalog number 2503 )
- Normal Human IgG (Sigma-Aldrich, catalog number: I2511 )
- p24Gag recombinant protein (Abcam, catalog number: ab43037 )
- Anti-p24Gag KC57 clone conjugated to fluorescein isothiocyanate (FITC) or to phycoerythrin also called RD1 (Beckman Coulter, catalog numbers: 6604665 and 6604667 )
- 1x phosphate-buffered saline (PBS) without calcium and magnesium (Corning, catalog number: 21-031-CV )
- Bovine serum albumin (BSA) (Axenia Biologix, catalog number: S200 )
- Triton X-100 (Thermo Fisher Scientific, catalog number: BP151-500 )
- 16% paraformaldehyde (PFA) solution (Electron Microscopy Sciences, catalog number: 15710 )
- Fluorescence-activated cell sorting (FACS) staining buffer (see Recipes)
Equipment
- Eppendorf centrifuge
- Rotating mixer
- 96-well V-bottom plate (optional) (Thermo Fisher Scientific, catalog number: 12-565-216 )
- Thermo Fisher lids for 96-well microplates (Thermo Fisher Scientific, catalog number: 14-245-53A )
- Plate sealers (VWR International, catalog number: 62402-921 )
- Eppendorf tubes
- Multichannel pipetman (optional)
- Pipetman
- Tips (1 ml, 200 µl, and 10 µl)
- Reagent reservoirs (optional)
- Tissue culture centrifuge for Eppendorf tubes or for 96 well plates (optional)
- 37 °C incubator
- Flow cytometer
Note: The FLAQ requires a flow cytometer equipped with a blue laser excitation (488 nm) and one measurement parameter. The photomultiplicator tube (PMT) with a 525/50 nm or a 585/42 band pass filter is used for the detection of FITC or RD1 (phycoerythrin) signals, respectively.
Software
- Forecyt (Intellicyt)
- FlowJoX software (Tree Star) or other FACS analysis software
Procedure
- Preparation of the FLAQ beads coated with p24Gag HIV-1 IIIB polyclonal antibodies
- Pipet 1 ml of Sphero Protein A Polystyrene Particles (referred hereafter as beads) into an Eppendorf tube.
- Centrifuge for 2 min at 12,000 rpm to pellet the beads.
- Remove supernatant.
- Add 1 ml of 1x PBS to beads pellet and centrifuge at 12,000 rpm for 2 min.
- Resuspend the beads pellet with 80 µl of the commercial vial of human anti-p24Gag HIV-1 IIIB polyclonal antibodies sold at the concentration 1 mg/ml. Pipet three times up and down and incubate for 15 min at room temperature on a rotating mixer.
- Centrifuge for 2 min at 12,000 rpm to pellet the beads.
- Remove and keep aside the antibody-containing supernatant.
- Add 1 ml of 1x PBS to the beads pellet.
- Centrifuge for 2 min at 12,000 rpm.
- Add another 1 ml of 1x PBS.
- Centrifuge for 2 min at 12,000 rpm.
- Add to the beads pellet 100 µl of a 1x PBS solution containing 4.8 mg/ml of Human normal IgG blocking reagent. Mix well by pipetting up and down 3 times.
- Incubate for 15 min at room temp on the rotating mixer.
- Centrifuge for 2 min at 12,000 rpm to pellet the beads.
- Discard the supernatant.
- Wash the beads twice with 1 ml of 10 mg/ml of BSA in PBS, spinning 2 min at 12,000 rpm each time.
- Resuspend the beads in 1 ml of 10 mg/ml of BSA in PBS and store these “FLAQ beads” at 4 °C.
- Repeat steps A1-17 using the supernatant containing p24Gag HIV-1 IIIB polyclonal antibodies from step 7 (Note 1).
- Pipet 1 ml of Sphero Protein A Polystyrene Particles (referred hereafter as beads) into an Eppendorf tube.
- Measuring p24Gag content in viral preparations
- Resuspend the Abcam p24Gag protein in FACS buffer at 8 µg/ml. Aliquot and freeze.
- Prepare 200 µl of the Abcam p24Gag protein standard by serial dilution ½ in FACS buffer with 0.5% Triton-X100. Typically we perform 8 serial dilutions ranging from 10 ng/ml to 78.12 pg/ml.
- Harvest viral supernatants and prepare dilutions in FACS buffer supplemented with 0.5% Triton-X100 (Note 2). 160 µl per sample will be required.
- Prepare a mix containing the following per sample:
0.5µl KC57 conjugated to RD1 or FITC
0.5 µl FLAQ beads
39 µl FACS buffer 0.5% Triton X-100 - Transfer 160 µl of the standards and the viral supernatants to a 96 well V-bottom plate.
- Add 40 µl of the reagent mix to each well and mix.
- Incubate at 37 °C for 1 h.
- Centrifuge each plate at 2,000 rpm for 2 min. Do not use plate sealers at this step to avoid well-to-well contamination due to the low superficial tension of the FACS buffer supplemented with 0.5% Triton X-100.
- Discard the supernatants and wash beads once with 150 µl of FACS buffer.
- Resuspend the beads in each well in 100 µl of FACS buffer containing 1% PFA.
- Acquire samples on a flow cytometer, which will create files with an FCS extension. FCS files are flow cytometry standard files containing all the specifications needed to completely describe experimental data flow sets.
- Analyze the fcs files to obtain the median fluorescence intensities of the beads by the FITC or PE detectors for experiments with KC-57 conjugated with FITC or RD1, respectively.
- Plot the median fluorescence in the PE channel (x axis) against the concentration of p24Gag in the standard (y axis) in Excel. After adding the trendline, determine the equation that fits the standard curve the best and calculate the concentration (y value) of the unknown.
- Resuspend the Abcam p24Gag protein in FACS buffer at 8 µg/ml. Aliquot and freeze.
Representative data
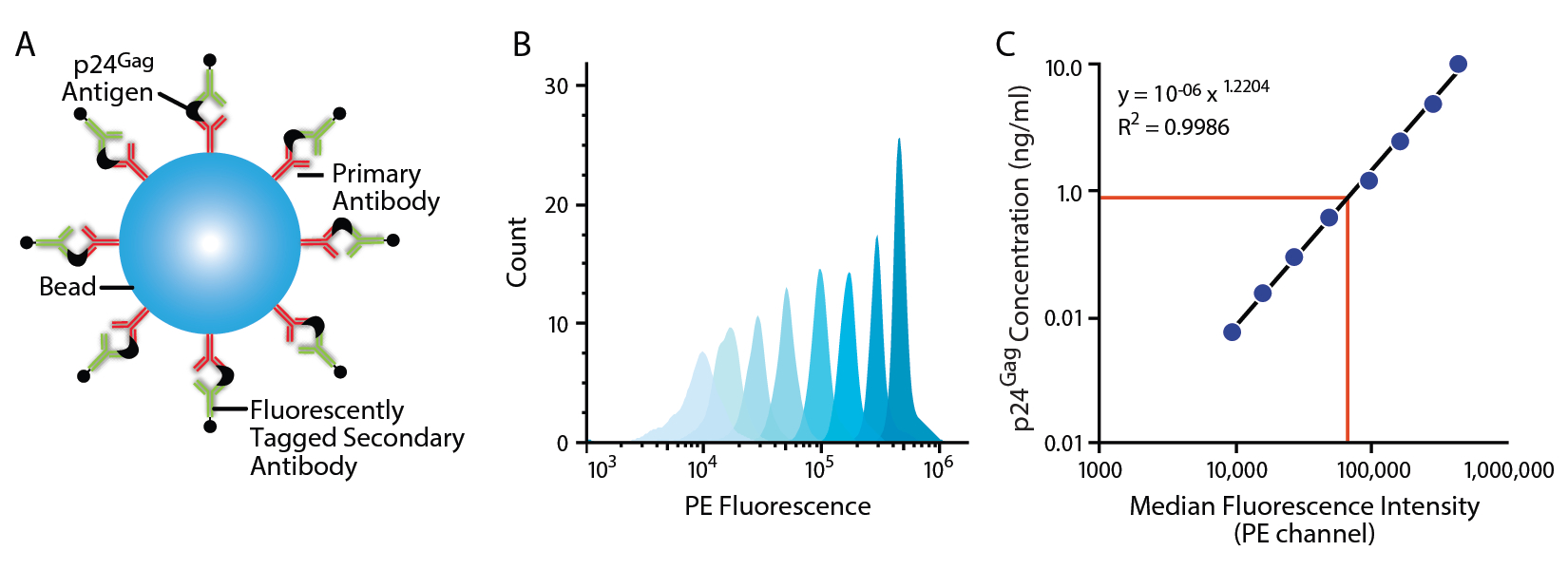
Figure 1. Representative data. A. Principle of the FLAQ assay. B. Gating strategy. The population of beads is first gated on Forward Scatter area (FSC-A) and Side Scatter area (SSC-A). Overlay of the population of beads for the standard (10 ng/ml to 78.12 pg/ml). C. Standard Curve.
Notes
- We can reuse the antibody at least 5 times, generating 5 ml of beads. The median fluorescence is slightly lower for each reuse.
- The presence of triton ensures good dilution of viral preparations. Supernatants from transfected 293T should contain between 500 and 1,000 ng/ml of p24Gag protein while supernatants from infected cultures (SupT1 or PBMCs) range from 20 to 200 ng/ml. Usually three dilutions per sample are analyzed to ensure that the measurement will fall within the dynamic range of the assay.
Recipes
- Fluorescence-activated cell sorting (FACS) staining buffer
1x phosphate-buffered saline (PBS)
2% fetal bovine serum
Stored at 4 °C
Acknowledgments
We would like to thank Hayden et al. for developing the FLAQ assay.
References
- Hayden, M. S., Palacios, E. H. and Grant, R. M. (2003). Real-time quantitation of HIV-1 p24 and SIV p27 using fluorescence-linked antigen quantification assays. AIDS 17(4): 629-631.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Gesner, M., Maiti, M., Grant, R. and Cavrois, M. (2014). Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24Gag. Bio-protocol 4(24): e1366. DOI: 10.21769/BioProtoc.1366.
Category
Microbiology > Microbe-host interactions > Virus
Immunology > Antibody analysis > Antibody-antigen interaction
Biochemistry > Protein > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










