- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Vanillin Assay of Arabidopsis Seeds for Proanthocyanidins
Published: Vol 4, Iss 23, Dec 5, 2014 DOI: 10.21769/BioProtoc.1309 Views: 12327
Reviewed by: Tie LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Targeting Ultrastructural Events at the Graft Interface of Arabidopsis thaliana by A Correlative Light Electron Microscopy Approach
Clément Chambaud [...] Lysiane Brocard
Jan 20, 2023 2981 Views

Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Chuanfeng Ju [...] Zhenqian Zhang
Mar 5, 2023 2053 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1181 Views
Abstract
Proanthocyanidins (PAs) are colorless flavonoid polymers and deposit in Arabidopsis seed coat specifically. Oxidation of PAs gives rise to brown color of mature seeds. PA accumulation can be affected by a number of growth conditions, such as temperature and sun light. PAs, which are converted from anthocyanidins, can protect seeds from outer environment and have a positive effect in seed longevity (Debeaujon, 2003). Vanillin turns red upon binding to leucoanthoanthocyanidins, catechins and monomers and terminal subunits of PAs (Butler et al., 1982; Deshpande et al., 1986). Based on this principle, PA deposition in Arabidopsis seed coat can be visualized.
Keywords: Seed coat colorMaterials and Reagents
- Arabidopsis seeds (Col-0 and tt mutants)
- 6 M HCl
- Sterile distilled water
- Dye solution (for coloration and reaction with PAs) containing 1% w/v vanillin (Sangon Biotech, catalog number: VT0974- 100g) and 6 M HCl stored in brown bottle
Note: The dye solution should be used after preparation as soon as possible (not longer than half an hour). - Vanillin reagent (see Recipes)
Equipment
- Brown bottle (or glass bottle covered with aluminum foil)
- 1.5 ml microcentrifuge tubes
- Glass slides (25.4 mm x 76.2 mm) and coverslips (20 mm x 20 mm)
- Dissecting needle and tweezer
- An SZ61-zoom stereomicroscope (OLYMPUS, model: SZ61)
- A compound light microscope (OLYMPUS, model: BX61 )
Procedure
- Intact seeds should be dipped into dye solution at room temperature.
- For mature seeds, the incubation should last for approximately 1 h, whereas for immature seeds, the incubation time can be up to 10 min, depending on the degree of immaturity. Figure 2 indicates silliques at different developmental stages.
- After the reaction with vanillin, seeds can be gently separated into embryos and seed coats with dissecting needle and tweezers under an SZ61-zoom stereomicroscope on glass slides.
- Cover slides with coverslips. A little bit extra vanillin reagent should be left to avoid drying (water and glycerol will fade this red color).
- Stained seed coats were observed and photographed with a compound light microscope.
Representative data
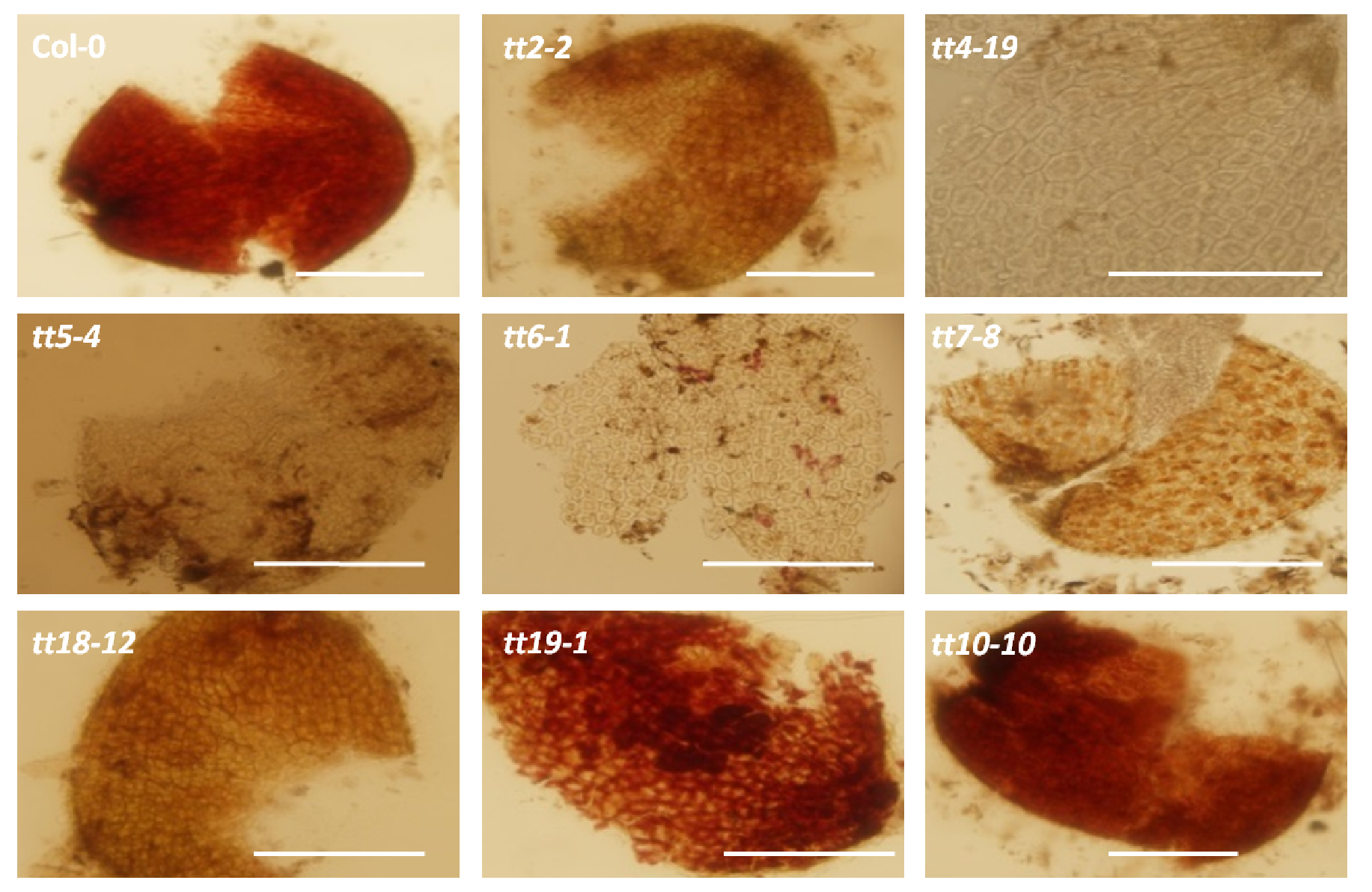
Figure 1. Comparison of the darkness of PA staining in seed coats among various test transparent mutants. Scale bars = 100 µm. (Wang et al., 2014) 
Figure 2. Comparison of Arabidopsis siliques between different developmental stages. Stage 1: Young siliques: less than or about one week after pollination. 10 min is enough for reaction between PAs and vanillin. Stage 2: Immature siliques: 1-3 weeks after pollination. 20 to 40 min are required for the reaction. Stage 3: Mature silique: 3 weeks or more after pollination, color of siliques appears pale yellow. 1 h is necessary for the reaction.
Notes
- Reaction between vanillin and PAs is affected by temperature. Make sure stain all the seeds at the same condition.
- Do not try to rinse seeds, especially young seeds, with water after dying with vanillin. Red color can be rinsed away by water.
- Make sure all the seeds were dipped into vanillin reagent at the same condition, and a quick centrifuge may help. For seed quantity, just cover the bottom of a 1.5 ml microcentrifuge tube (one or two layers).
Recipes
- Vanillin reagent
The vanillin reagent should be fresh and 1 ml is quite enough for seed staining. Make sure vanillin reagent volume and seed number are the same in wildtype and mutant microcentrifuge tubes. Stained red color continues getting dark along with time duration. It is very important to photograph seed coats at the same light condition. Light intensity can affect visual effects.
Acknowledgments
The work was sponsored by the Natural Science Foundation of China (Grant nos. 31171463). Our protocol is an improvement based on a method described by Hagerman (2002) with sorghum grains.
References
- Butler, L. G., Price, M. L. and Brotherton, J. E. (1982). Vanillin assay for proanthocyanidins (condensed tannins): modification of the solvent for estimation of the degree of polymerization. J Agr Food Chem 30(6): 1087-1089.
- Debeaujon, I., Nesi, N., Perez, P., Devic, M., Grandjean, O., Caboche, M. and Lepiniec, L. (2003). Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell 15(11): 2514-2531.
- Deshpande, S. S., Cheryan, M. and Salunkhe, D. K. (1986). Tannin analysis of food products. Crit Rev Food Sci Nutr 24(4): 401-449.
- Hagerman, A. E. (2002). Vanillin assay. The Tannin Handbook.
- Wang, Z., Chen, M., Chen, T., Xuan, L., Li, Z., Du, X., Zhou, L., Zhang, G. and Jiang, L. (2014). TRANSPARENT TESTA2 regulates embryonic fatty acid biosynthesis by targeting FUSCA3 during the early developmental stage of Arabidopsis seeds. Plant J 77(5): 757-769.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Xuan, L., Wang, Z. and Jiang, L. (2014). Vanillin Assay of Arabidopsis Seeds for Proanthocyanidins. Bio-protocol 4(23): e1309. DOI: 10.21769/BioProtoc.1309.
Category
Plant Science > Plant biochemistry > Other compound
Plant Science > Plant physiology > Tissue analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









