- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Characterisation of Dendritic Cells from Peripheral Blood
Published: Vol 4, Iss 22, Nov 20, 2014 DOI: 10.21769/BioProtoc.1300 Views: 17451
Reviewed by: Jia LiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
Nov 5, 2025 1449 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Latency and reactivation of human cytomegalovirus (HCMV) is intimately associated with the myeloid lineage. Multiple studies have used in vitro protocols to generate dendritic cells (DCs) from myeloid precursors. Here we describe the direct isolation of DCs from peripheral blood to study HCMV latency directly in this cell type.
Keywords: Dendritic cellMaterials and Reagents
- 100 ml peripheral blood obtained via subcutaneous venepuncture
- Heparin - to prevent clotting of blood
- Histopaque-1077 (Sigma-Aldrich, catalog number: 10771 ) or any equivalent ficoll solution (density 1.077 g/ml)
- Fetal calf serum (FCS)
- PBS (chilled)
- DC isolation kit (Miltenyi Biotec, catalog number: 130-094-487 )
- Antibodies (BD biosciences unless stated otherwise)
- MACS buffer (see Recipes)
Equipment
- Magnetic-activated cell sorting (MACS) magnet
- LS columns (Miltenyi Biotec, catalog number: 130-042-401 )
- Centrifuge (50 ml tube capacity)
- 50 ml polypropylene conical tubes (FalconTM)
- 14 ml snap-cap polypropylene tubes
Procedure
- Mononuclear cell isolation
- Cool MACS magnet adaptor and LS column at 4 °C. LS columns have a total loading capacity of 2 x 109 unpurified cells (which would allow the isolation of 108 labelled cells).
- Cool MACS Buffer and PBS on ice.
- Dilute the blood 1:1 in PBS.
- Add 15 ml histopaque-1077 (density 1.077 g/ml) to a 50 ml conical tube.
- Carefully layer 30-35 ml of blood/PBS on top.
- Spin at 800 x g for 20 min at RT. No brake on centrifuge.
- Take WBC at interphase. To do this use a Pasteur pipette. Squeeze the teat prior to adding to Falcon and then suck up the white fluffy looking layer resting on histopaque. Avoid aspirating excessive histopaque-1077 as it can prevent pelleting of cells.
- Pellet cells at 300 x g for 10 min (RT). If there is a problem pelleting cells possibly due to contaminating lymphoprep then dilute a further 1:4 in PBS and repeat centrifugation step.
- Wash in cold PBS 300 x g for 10 min. Add 50 ml of PBS to pelleted cells.
- Count cells. May need to dilute (1:100). A sample is taken as pre-sort control for FACs analysis to determine enrichment.
- Centrifuge at 300 x g for 10 min (RT).
- Re-suspend pellet in 300 µl of MACS buffer per 108 total cells. So for 3 x 109 the cells would be re-suspended in 9 ml. Add 100 µl of FcR Blocking reagent (in kit) per 108 and 100 µl of Microbead dendritic antibody cocktail.
- Incubate for 10 min at 4 °C.
- Transfer to a 50 ml Falcon and wash in PBS. Spin 300 x g for 10 min (RT).
- As spin ends, charge the column.
- To charge the column insert LS column into magnet. Then run 3 ml of MACS buffer through the column. Discard flow through.
- Re-suspend the centrifuged cells in 500 µl of MACS buffer per 108 cells and add to column.
- Collect flow through. Re-apply this flow through to the column. Collect again and then wash the column a further two times with 3 ml washes. The flow through represents the dendritic cell population.
- Count cells.
- Cells are then used for downstream application.
- Cool MACS magnet adaptor and LS column at 4 °C. LS columns have a total loading capacity of 2 x 109 unpurified cells (which would allow the isolation of 108 labelled cells).
- FACS staining
- Using the cell count above. 105 cells are pelleted (400 x g for 5 min) in FACs tubes and re-suspended in residual PBS in tube (approximately 50 µl).
- Add 1 µl of normal mouse serum to cells and incubate for 10 min (RT).
- Proceed directly to adding directly conjugated antibody to cells (or isotype matched controls) as dictated by antibody datasheet. Stain in presence of normal serum. Stain for 20 min at RT in the dark (unless IgM which is then done at + 4 °C). Have one tube unstained for setting the parameters for collection.
- Wash cells in 10x PBS and then spin (400 x g; 5 min, RT). Resuspend in 500 µl and proceed to FACs analysis.
- Cells are characterized by the expression of a panel of cell surface markers consistent with a DC phenotype. The cells should be HLA-DRhigh whilst TCR, CD14 and CD19 negative. Cells will also express CD1a, CD86 and CD83low.
- Using the cell count above. 105 cells are pelleted (400 x g for 5 min) in FACs tubes and re-suspended in residual PBS in tube (approximately 50 µl).
Representative data

Figure 1. Isolation of PBMC on histopaque 1077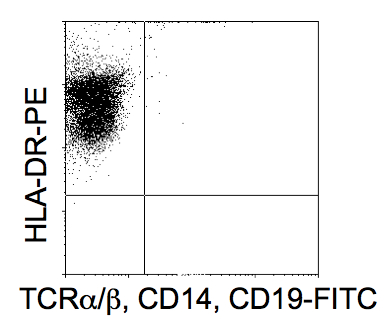
Figure 2. FACs analysis of putative DCs post isolation shown to be HLA-DR positive and CD14, 19 and TCRα/β negative
Recipes
- MACS buffer
PBS supplemented with 0.5% fetal calf serum and 2 mM EDTA
References
- Reeves, M. B. and Sinclair, J. H. (2013). Circulating dendritic cells isolated from healthy seropositive donors are sites of human cytomegalovirus reactivation in vivo. J Virol 87(19): 10660-10667.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Reeves, M. and Sinclair, J. (2014). Isolation and Characterisation of Dendritic Cells from Peripheral Blood. Bio-protocol 4(22): e1300. DOI: 10.21769/BioProtoc.1300.
Category
Microbiology > Microbe-host interactions > Virus
Cell Biology > Cell-based analysis > Flow cytometry
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link