- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Expression and Purification of the Eukaryotic MBP-MOS1 Transposase from Sf21 Insect Cells
Published: Vol 4, Iss 20, Oct 20, 2014 DOI: 10.21769/BioProtoc.1262 Views: 11657
Reviewed by: Longping Victor TseAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Prokaryotic Expression and Purification of the hSox2-HMG Domain
Lijie Yang [...] Jingjun Hong
Aug 20, 2025 2377 Views

An Optimized Enzyme-Coupled Spectrophotometric Method for Measuring Pyruvate Kinase Kinetics
Saurabh Upadhyay
Aug 20, 2025 2455 Views

Cluster FLISA—A Method to Compare Protein Expression Efficiency Between Cell Lines and Subunit Clustering of Proteins
Sabrina Brockmöller and Lara Maria Molitor
Nov 5, 2025 1209 Views
Abstract
Here, we present the full-length protocol for purifying the recombinant MOS1 transposase from insect cells used in our recent publication (Pflieger et al., 2014), which involved a N-terminal MBP-tag and maltose-affinity chromatography.
Due to their overall basic properties, transposases are often difficult to purify, especially because they tend to aggregate. Since the 90s, we chose a method of purification without a denaturation step. Our first priority was to preserve the 3D structure of the protein in order to maintain its biochemical activities with the highest specific activity. Nevertheless, our production/purification made from bacteria regularly contain truncated products (or degradation products) and their levels increase with concentration of purified transposase. In contrast, production/purification made from eukaryotic cells do not contain such degradation product. We thus developed a protocol involving the pVL1392 baculovirus transfer vector and the BaculoGoldTM baculovirus expression system, allowing the expression of recombinant MOS1 from baculovirus-infected Sf21 cells.
Materials and Reagents
- Sf21 cells (Life Technologies, Gibco®, catalog number: 11497 )
- Insect Express Sf9/S2 medium (PAA, catalog number: E15-875 )
- Fetal bovine serum (Life Technologies, Gibco®)
- Baculogold baculovirus expression system (BD Biosciences, catalog numbers: 554739 and 560129 )
- Anti-MBP anti-body (Santa Cruz, catalog number: sc-808 )
- Tris.HCl (pH 7.6)
- NaCl
- DTT
- NP40
- Antiprotease cocktails (like Roche’s EDTA-free cocktail tablets, catalog number: 11-873 580-001 )
- Maltose
- Bovine serum albumin (BSA)
- Lysis buffer (see Recipes)
- Binding buffer (see Recipes)
- Elution buffer (see Recipes)
Equipment
- 25 cm2, 150 cm2 flask (usual culture-cell equipment)
- 1 ml prepacked column (GE Healthcare, MBPTrap™ HP) or 1 ml of amylose resin in batch (New England Biolabs)
Procedure
- Sf21 culture conditions
- Sf21 cells are cultivated in Insect Express Sf9/S2 medium (PAA) containing 5% fetal bovine serum (or FBS, PAA) at 27 °C (herein called “complete media”).
- Every 4 to 6 days, a new 25 cm2 flask is inoculated with 1 to 1.25 x 106 viable cells in 5 ml of complete medium.
- Sf21 cells are cultivated in Insect Express Sf9/S2 medium (PAA) containing 5% fetal bovine serum (or FBS, PAA) at 27 °C (herein called “complete media”).
- Generating a recombinant baculovirus that expresses the MBP-MOS1 fusion protein in Sf21 cells
- The MBP-MOS1 coding sequence is cloned into the pVL-1392 at the SmaI restriction site.
- High-titre, low-passage recombinant Baculovirus stocks are obtained in Sf21 cells following the instructions of the “Baculogold baculovirus expression system” manufacturer (BD Biosciences) (https://www.bdbiosciences.com/documents/Baculovirus_vector_system_manual.pdf).
- 5 to 10 batches of recombinant Baculoviruses are assayed for their ability to produce the recombinant MBP-MOS1 in infected Sf21 cells by Western blot (https://www.sciencepub.net/researcher/0102/09_1259_yang_west_research0102.pdf) with the anti-MBP anti-body (dilution 1/10,000). The most efficient isolate is selected for further protein production processes.
- The MBP-MOS1 coding sequence is cloned into the pVL-1392 at the SmaI restriction site.
- Producing the MBP-MOS1 fusion protein in Sf21 cells
- The selected baculovirus stock is used to infect Sf21 cells.
- Twenty-one 150 cm2 flasks with 6 x 107 viable cells per flask in 30 ml of complete medium are prepared and incubated at 27 °C until the cells reach confluence (see Figure 1).
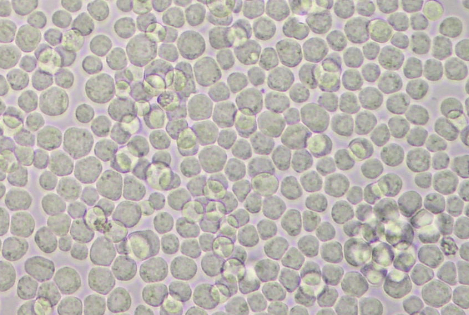
Figure 1. Confluent non-infected Sf21 cells
- The medium is then replaced by 10 ml of medium without FBS and cells are infected with 100 µl of high titre recombinant Baculoviruses stock solution (virus titer should be 1 to 2 x 108 pfu/ml).
- Cells are incubated for 3 h at 27 °C and then 5 ml of complete medium (with 5% FBS) is added. The cells are incubated for 3 days at 27 °C, in the dark.
- Check for signs of infection 2-3 days after inoculation. Cells should be enlarged in size (about 2 fold) and a large nucleus should be visible (see Figure 2).
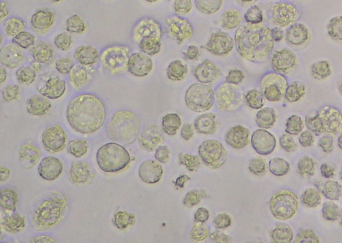
Figure 2. Infected Sf21 cells
- To pellet cells, gently dislodge cells from monolayers by smacking and transfer the cell suspension to a sterile centrifuge tube of appropriate size. Spin down cells at 10,000 x g for 5 min. The MBP-MOS1 protein will be found in the cell pellet, which can be stored at -80 °C or immediately processed.
- The selected baculovirus stock is used to infect Sf21 cells.
- MBP-MOS1 fusion protein extraction in crude extracts
- Pellets are washed in 1x PBS and pooled so that one pellet contains the cells coming from three 150 cm2 flasks.
- Cytoplasmic crude extract (CCE).
- Each pellet is resuspended in 3.5 ml of lysis buffer.
- Cells are lysed on ice for 30 min and then centrifuged at 15,000 x g and 4 °C for 30 min. The supernatant (≈30 ml) corresponds to the cytoplasmic crude extract (CCE).
- Protein concentration is usually around 5 to 10 µg/µl (i.e. a total amount of about 125 to 250 mg), as determined by Bradford assay (http://web.mnstate.edu/provost/bradfordproteinassayprotocol.pdf) using BSA as a standard. Keep the CCE on ice.
- Each pellet is resuspended in 3.5 ml of lysis buffer.
- Nuclear crude extract (NCE)
- Each pellet is resuspended in 0.75 ml of lysis buffer without NP40.
- Sonicated on ice (Ultrasonic Processor, output power at 100%, 3 pulses of 15 sec each) and centrifuged at 10,000 x g and 4 °C for 10 min. The supernatant (≈ 6 ml) corresponds to the nuclear crude extract (NCE).
- Protein concentration is usually around 5 to 10 µg/µl (i.e. a total amount of about 30 to 50 mg). Keep the NCE on ice.
- Each pellet is resuspended in 0.75 ml of lysis buffer without NP40.
- Pellets are washed in 1x PBS and pooled so that one pellet contains the cells coming from three 150 cm2 flasks.
- MBP-MOS1 fusion protein purification
CCE and NCE previously obtained can be processed either separately or pooled. In the following, CCE and NCE are pooled together, giving a whole cellular crude extract. MBP-MOS1 can be purified by two ways: Using a 1 ml prepacked column or 1 ml of amylose resin in batch.- Prepacked column
- Equilibrate the column with at least 5 ml of binding buffer at 0.5 ml/min.
- Adjust the crude extract concentration to 1 mg/ml with the NCE lysis buffer. Apply the sample.
- Wash with at least 10 ml of binding buffer (until no material appears in the flow-through). Elute with 5 ml of elution buffer, and collect into 0.5 ml fractions.
- After one column volume (about 1 ml), the peak of purified MBP-MOS1 is eluted within two to three fractions, corresponding to those having the maximum UV absorbance at 280 nm.
- Pool the positive fractions and store as 50 µl aliquots at -80 °C.
- Equilibrate the column with at least 5 ml of binding buffer at 0.5 ml/min.
- Amylose resin in batch
- Wash the resin with at least 5 ml of binding buffer. Add 1 ml of resin to the crude extract.
- Incubate for 1 h at 4 °C with gentle mixing. Centrifuge at 800 x g and 4 °C for 5 min. Discard the supernatant.
- Wash the resin pellet with at least 10 ml of binding buffer. Centrifuge at 800 x g and 4 °C for 5 min. Discard the supernatant. This is to be repeated five times.
- Elute the MPB-MOS1 with 1 ml of elution buffer; Centrifuge at 800 x g and 4 °C for 5 min. Recover the supernatant (E1 fraction).
- Elution can be repeated twice, giving E2 and E3 fractions.
- Highly concentrated fractions (E1 and often E2) are stored as 50 µl aliquots at -80 °C.
- MBP-MOS1 concentration in positive fractions (from both methods) is determined by Bradford assay using BSA as a standard.
- From twenty F1 50 cm2 infected flasks, 1-2 ml at about 0.5 to 1 µg/µl is expected, depending on the efficiency of the infection.
- Wash the resin with at least 5 ml of binding buffer. Add 1 ml of resin to the crude extract.
- Prepacked column
Recipes
- Lysis buffer
20 mM Tris.HCl (pH 7.6)
100 mM NaCl
1 mM DTT
1% NP40
Antiprotease cocktails (according to the manufacturer’s recommendations)
- Binding buffer
20 mM Tris.HCl (pH 9)
100 mM NaCl
1 mM DTT
- Elution buffer
Binding buffer containing 10 mM maltose
Acknowledgments
Dussassois-Montagne was a doctoral fellow of the French government (MRT). This work was supported by the Région Centre (InhDDE project), the French ANR (Elegineer project), the University of Tours, and the Centre National de la Recherche Scientifique.
References
- Pflieger, A., Jaillet, J., Petit, A., Auge-Gouillou, C. and Renault, S. (2014). Target capture during Mos1 transposition. J Biol Chem 289(1): 100-111.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Jaillet, J., Dussaussois-Montagne, A., Renault, S. and Augé-Gouillou, C. (2014). Expression and Purification of the Eukaryotic MBP-MOS1 Transposase from Sf21 Insect Cells. Bio-protocol 4(20): e1262. DOI: 10.21769/BioProtoc.1262.
- Pflieger, A., Jaillet, J., Petit, A., Auge-Gouillou, C. and Renault, S. (2014). Target capture during Mos1 transposition. J Biol Chem 289(1): 100-111.
Category
Molecular Biology > Protein > Expression
Biochemistry > Protein > Expression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link