- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fitness Measurements of Evolved Esherichia coli
Published: Vol 4, Iss 17, Sep 5, 2014 DOI: 10.21769/BioProtoc.1228 Views: 12592
Reviewed by: Fanglian HeKanika Gera

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vitro DNA Polymerization Activity Assay Using Cell-free Extracts
Anurag K. Sinha and Malay K. Ray
Aug 20, 2014 9249 Views

Introduction and Sequencing of Patient-isolated HBV RT Sequences into the HBV 1.2-mer Replicon
Sung Hyun Ahn [...] Kyun-Hwan Kim
Apr 20, 2015 9696 Views

Estimation of the Minimum Number of Replication Origins Per Chromosome in any Organism
Marcelo S. da Silva
Oct 20, 2020 4214 Views
Abstract
Bacteria can adapt very rapidly to novel selective pressures. In the transition from commensalism to pathogenicity bacteria have to face and adapt to the host immune system. Specifically, the antagonistic interaction imposed by one of the first line of defense of innate immunity cells, macrophages, on commensal bacteria, such as Escherichia coli (E. coli), can lead to its rapid adaptation. Such adaptation is characterized by the emergence of clones with mutations that allow them to better escape macrophage phagocytosis. Here, we describe how to quantify the amount of fitness increase of bacterial clones that evolved under the constant selective pressure of macrophages, from a murine cell line RAW 264.7. The most widely used assay for measuring fitness changes along an evolutionary laboratory experiment is a competitive fitness assay. This assay consists of determining how fast an evolved strain outcompetes the ancestral in a competition where each starts at equal frequency. The strains compete in the same environment of the evolution experiment and if the evolved strain has acquired strong beneficial mutations it will become significantly overrepresented in repeated competitive fitness assays.
Keywords: Experimental evolutionMaterials and Reagents
- RAW 264.7 murine macrophage cell line (MΦs)
- E. coli strains, marked with constitutive expression of yellow (YFP) and cyan (CFP) fluorescent proteins (e.g. E. coli- MC4100, galK::CFP/YFP, AmpRStrepR)
- RPMI media1640 (Life Technologies, Gibco®, catalog number: 11875-093 )
- 2-mercaptoethanol solution (Life Technologies, InvitrogenTM, catalog number: 31350-010 )
- 1 M HEPES buffer (Life Technologies, InvitrogenTM, catalog number: 15630-056 )
- 100 mM sodium pyruvate (Life Technologies, InvitrogenTM, catalog number: 11360-039 )
- Heat-inactivated fetal bovine serum (FBS)
- 200 mM L-glutamine (Life Technologies, Gibco®, catalog number: 25030-081 )
- 10,000 U/ml penicillin/streptomycin (Life Technologies, Gibco®, catalog number: 15140-122 )
- Streptomycin sulfate salt (Sigma-Aldrich, catalog number: S9137 )
- 50 mg/ml Gentamycin solution (Sigma-Aldrich, catalog number: G1397 )
- 1x Phosphate-buffered saline (PBS)
- Trypan blue solution (Sigma-Aldrich, catalog number: T8154 )
- CpG-ODN -1826 (Sigma-Aldrich) (5´-TCCATGACGTTCCTGACGTT-3´)
- RPMI-complete media (see Recipes)
- RPMI-strep media (see Recipes)
Equipment
- 37 °C, 5% CO2 cell culture incubator
- Microscope
- Centrifuge (Sigma-Aldrich, model: 4K15 with a rotor 11150)
- Neubauer cell counting chamber
- Air flow chamber
- 12-well microtiter plates
- Cell culture flasks (25 cm2 and 75 cm2 growth area)
- 15 ml Falcons tubes
- Cell scrapers
- 2 μm size beads (Sphero AccuCount Blank Particles)
- Flow cytometer
Procedure
- Grow MΦs (in the 37 °C, 5% CO2 incubator) in 75 cm2 culture flask in RPMI- complete media until 80% confluency (Note 1).
- Change old media (remove old media by aspiration and add fresh RPMI-complete media) and detach Mϕs with a cell scraper.
- Carefully pipette culture up and down until pellet is dissolved.
- Centrifuge in 15 ml Falcon tubes at 1,200 rpm for 5 min (Note 2).
- Remove supernatant and re-suspend pellet in the same volume of RPMI-Strep media (Note 3).
- Count MΦs in the Neubauer cell counter with Trypan blue dye (use 10 μl of cell suspension with 10 μl of dye). Allow the dye to stain for approximately 5 to 15 min. If cells are exposed to extended period of time to this dye, viable cells, as well as nonviable cells, may begin to uptake dye.
- Approximately 0.7-1.3 x 106 cells/ml activate with CpG-ODN at a final concentration of 2 µg/µl for 24 h in 10 cm2 flask (8 ml total volume) (Note 4).
- Grow separately evolved E. coli - CFP (or YFP) and ancestral E. coli - YFP (or CFP) bacterial populations from the frozen stock in RPMI-Strep media (3 ml of the total volume for each well) in the 12-well plates for 24 h (for the evolved E. coli - CFP (or YFP) please see our other protocol “Evolution of Escherichia coli to Macrophage Cell Line” (Miskinyte and Gordo, 2014).
- After 24 h repeat steps 2-6, but for the step 4 use 1,000 rpm instead of 1,200.
- Seed 0.8-1.6 x 106 cells/ml in 12-well microtiter plates (3 ml total volume for each well).
- Allow cells to attach in the 37 °C, 5% CO2 incubator for 2 h.
- Mix E. coli - CFP (evolved) with E. coli - YFP (ancestral) at a ratio of 1 to 1.
- Count numbers of CFP (Nie) and YFP (Nia) bacteria before competition in the mixture by using 2 μm size beads in the flow cytometer (Note 5).
- Wash MΦs with RPMI-Strep media (remove old media by aspiration and add fresh RPMI-Strep media).
- Infect MΦs at the multiplicity of infection (MOI) of 1 to 1 (mixture of 106 bacteria/ml to 106 MΦs/ml).
- After 24 h of infection, detach MΦs with a cell scraper and centrifuge culture at 4,000 rpm for 10 min. This procedure lyses MΦs and releases intracellular bacteria (Note 6).
- Discard the supernatant and resuspend the pellet in 3 ml of PBS.
- Dilute bacteria in PBS and count CFP (Nfe) and YFP (Nfa) bacterial numbers after competition in Flow cytometer.
- Use Formula 1 to calculate relative fitness increase of the evolved bacteria over the ancestral.
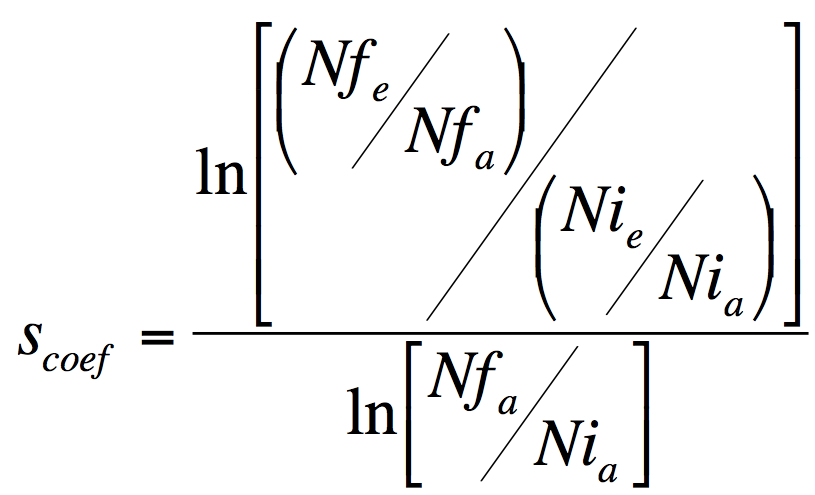
Formula 1. Selection coeficient, a measure of relative fitness increase. Scoef is the selective advantage of the evolved strain e over the ancestral strain a, Nfe and Nfa are the numbers of evolved (e) and ancestral (a) bacteria after competition and Nia and Nie are the initial numbers, before the competition.
Notes
- You should compare the amount of space covered by cells with unoccupied spaces to estimate approximately percent confluency (check: http://www.abcam.com/ps/pdf/protocols/cell culture.pdf).
- If the MΦ culture has many dead (aggregated or detached cells) centrifuge at 800 rpm. This way healthy cells will pellet at the bottom of the tube and dead cells will remain mainly in the supernatant.
- Streptomycin is used to reduce the possibility of contamination. The strain of E. coli is STR resistant. If you use a different antibiotic, test for its toxicity on the MΦs cell line.
- This dilution is necessary because MΦs will grow in 24 h. We use CpG-ODN-1826, because ODN 1826 is a B-class CpG ODN specific for mouse TLR9 that leads to strong immunostimulatory effects after 24 h of activation. If you intend to use other cell line (e.g. ODN 2395 is specific for class C human TLR9) choose an appropriate ODN.
- You can use the following dilution to count in the flow cytometer: 10 µl of bacterial culture (diluted 1: 100), 10 µl of beads and 180 ml PBS.
- You can also detach MΦs, by bending a blue 1,000 µl pipette tip and using it as a scraper. Remember that for each replicate well you need to use a new sterile scraper.
Recipes
- RPMI-complete media
500 ml RPMI 1640
50 ml FBS
5 ml sodium pyruvate
5 ml HEPES
5 ml L-glutamine
0.5 ml 2-mercaptoethanol solution
0.5 ml gentamycin solution
5 ml penicillin/streptomycin solution
Stored at 4 °C
- RPMI-strep media
500 ml RPMI 1640
50 ml FBS
5 ml sodium pyruvate
5 ml HEPES
5 ml L-glutamine
0.5 ml 2-mercaptoethanol solution
1 ml of 50 mg/ml streptomycin solution
Stored at 4 °C
Acknowledgments
This protocol was adapted or modified from Richard E. Lenski, Michael R. Rose, Suzanne C. Simpson and Scott C. Tadler 1991, Long-Term Experimental Evolution in Escherichia coli. I. Adaptation and Divergence During 2,000 Generations. The American Naturalist, Vol. 138, No. 6, pp. 1315-1341. The research received funding from the European Research Council under the European Community's Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement no 260421 – ECOADAPT. IG acknowledges the salary support of LAO/ITQB & FCT.
References
- Lenski, R. E., Rose, M. R., Simpson, S. C. and Tadler, S. C. (1991). Long-term experimental evolution in Escherichia coil. I. Adaptation and divergence during 2,000 generations. American Naturalist 138:1315-1341.
- Miskinyte, M. and Gordo, I. (2014). Evolution of Escherichia coli to macrophage cell line. Bio-protocol 4(17): e1227.
- Miskinyte, M., Sousa, A., Ramiro, R. S., de Sousa, J. A., Kotlinowski, J., Caramalho, I., Magalhaes, S., Soares, M. P. and Gordo, I. (2013). The genetic basis of Escherichia coli pathoadaptation to macrophages. PLoS Pathog 9(12): e1003802.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Miskinyte, M. and Gordo, I. (2014). Fitness Measurements of Evolved Esherichia coli. Bio-protocol 4(17): e1228. DOI: 10.21769/BioProtoc.1228.
- Miskinyte, M., Sousa, A., Ramiro, R. S., de Sousa, J. A., Kotlinowski, J., Caramalho, I., Magalhaes, S., Soares, M. P. and Gordo, I. (2013). The genetic basis of Escherichia coli pathoadaptation to macrophages. PLoS Pathog 9(12): e1003802.
Category
Microbiology > Microbe-host interactions > Bacterium
Microbiology > Microbial genetics > DNA > DNA replication
Molecular Biology > DNA > Genotyping
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link