- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of ILC2 from Mouse Liver
Published: Vol 4, Iss 14, Jul 20, 2014 DOI: 10.21769/BioProtoc.1179 Views: 13151
Reviewed by: Savita NairAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Group 2 innate lymphoid cells (ILC2) are a recently characterized cell population which lacks specific antigen receptors and contributes to immune responses at mucosal surfaces of lung and gut. Recently, we demonstrated that ILC2 expand in the context of chronic liver diseases in mice and contribute to pathology in an IL-33 dependent manner. Here, we describe a protocol to isolate highly purified ILC2 from mouse livers. This procedure provides effective digestion of liver tissue and limits proteolytic degradation of cell surface receptors leading to increased yields of biologically functional cells. The number of liver resident ILC2 in the steady state is low, however their number dramatically increase upon systemic or local treatment of mice with cytokines such as IL-25 and IL-33. Using minicircle-based expression constructs for these cytokines high numbers of functional ILC2 can be isolated with the protocol provided here.
Keywords: Group 2 innate lymphoid cellsMaterials and Reagents
- Liver from a IL-33 or IL-25 treated mouse (e.g. C57BL/6 or Balb/c)
- Fetal calf serum (FCS)
- RPMI-1640 medium
- Easycoll separation solution (density 1.124) (Biochrom, catalog number: L6145 )
- Anti-mouse CD16/CD32 (Fc-Block) (eBioscience, catalog number: 14-0161 )
- 0.5 M CaCl2 solution
- 0.2 M MgCl2 solution
- AutoMACS rinsing solution (Miltenyi Biotec)
- Brilliant Violet 421 Streptavidin (BioLegend, catalog number: 405226 )
- Antibodies
- FACS antibodies
- Anti-mouse lineage-antibodies biotin labelled
- Anti-CD3 (clone 145-2C11) (eBioscience, catalog number: 13-0031 )
- Anti-CD45R (clone RA3-6B2) (eBioscience, catalog number: 13-0452 )
- Anti-Ly-6G (clone RB6-8C5) (eBioscience, catalog number: 13-5931 )
- Anti-CD11b (clone M1/70) (eBioscience, catalog number: 13-0112 )
- Anti-NK1.1 (clone PK136) (eBioscience, catalog number: 13-5941 )
- Anti-Ter-119 (clone Ter-119) (eBioscience, catalog number: 13-5921 )
- Anti-SiglecF (clone ES22-10D8) (Miltenyi Biotec, catalog number: 130-101-861 )
- Anti-CD5 (clone 53-7.3) (Miltenyi Biotec, catalog number: 130-101-960 )
- Anti-CD3 (clone 145-2C11) (eBioscience, catalog number: 13-0031 )
- Krebs-Ringer-Buffer (KRB) (see Recipes)
- Collagenase IV solution (Sigma-Aldrich, catalog number: C5138 ) (see Recipes)
- DNase I solution (Roche Diagnostics, catalog number: 10104159001 ) (see Recipes)
- 30% Biocoll (see Recipes)
- 80% Biocoll (see Recipes)
- FACS buffer (see Recipes)
- ACK buffer (see Recipes)
- PEB buffer (see Recipes)
Equipment
- GentleMACS C tube (Miltenyi Biotec)
- 6 well plates
- GentleMACS dissociator (Miltenyi Biotec)
- MACSmix tube rotator (Miltenyi Biotec)
- Centrifuge (Thermo Fisher Scientific, model: Multifuge X1R )
- Cell sorter (e.g. FACSaria, BD Biosciences)
- 100 µm cell strainer (Corning, catalog numer: 08-771-19 )
- Hemocytometer
- 15 ml Falcon tubes
- 50 ml Falcon tubes
- FACS tubes
Procedure
- Freshly prepare liver digestion solution by pipetting 4.4 ml Krebs-Ringer-Buffer (KRB), 25 µl DNase I solution, 500 µl Collagenase IV solution, 20 µl 0.5 M CaCl2 and 50 µl 0.2 M MgCl2 into a gentleMACS C tube. Warm up by keeping at 37 °C for 30 min. This solution ensures effective liver tissue digestion while proteolytic degradation of immune cell surface receptors is limited.
- Sacrifice mouse by cervical dislocation and remove the liver from a mouse aseptically under the sterile bench and transfer the organ into a well of a sterile 6 well plate filled with 5 ml KRB buffer. If co-isolation of some ILC2 cells potentially present in the vasculature should be avoided include liver perfusion e.g. via the portal vein system using standard techniques described elsewhere.
- Rinse the liver with KRB buffer and transfer it into the C tube containing pre-warmed liver digesting solution.
- Dissociate the tissue by applying the C tube into the gentleMACS Dissociator and running the program m_liver-01.02.
Note: One mouse liver can be proceeded per C tube, the program can be run 2-3 times till the tissue is fully homogenized.
- Attach the C tube containing the homogenized tissue to the MACSmix Tube Rotator and incubate the sample at 37 °C for 30 min under continuous rotation.
- Apply the tube to the gentleMACS Dissociator again and run the program m_liver-02.02.
- Prepare a 50 ml tube and a 100 µm cell strainer by rinsing them with PEB buffer.
- Apply the homogenized tissue from C tube on the strainer on the 50 ml tube and wash the strainer with additional 10 ml PEB buffer.
- Discard the cell strainer and add further 20 ml PEB buffer to the tube. Centrifuge the sample at 20 x g at 4 °C for 4 min. This step removes unwanted hepatocytes from the liver cell suspension.
- Carefully remove the supernatant from the sample and transfer it into a new 50 ml tube.
- Wash the cells by adding 30 ml PEB buffer and centrifugation at 300 x g for 10 min at 4 °C.
- Carefully discard the supernatant and resuspend the cell pellet in 1 ml of PEB buffer.
- Apply 10 ml of ACK buffer on the suspension to remove red blood cells. Incubate the sample at room temperature for 5 min.
- Wash the sample two times by adding 30 ml PEB buffer and centrifugation at 300 x g for 10 min at 4 °C.
- Fill 15 ml tube with 5 ml of 80% Biocoll separation solution.
- Resuspend isolated cells in 10 ml 30% Biocoll separation solution and overlay slowly to the 80% Biocoll solution. Centrifuge the cells at room temperature with 1,400 x g for 20 min without breaks.
- Collect the target cells by carefully pipetting up the interface fraction between the gradients into a new 50 ml tube.
- Wash with 30 ml PEB buffer and centrifuge at 300 x g for 15 min.
- Count the cells by using a hemocytometer and distribute to FACS tube for staining.
- Prior to staining block unspecific bindings by incubating cells with 1 µl/107 cells of Fc-Block at 4 °C for 5 min.
- Proceed immediately to staining using the following antibodies using the manufacturer’s recommended concentrations: anti-Sca-1, anti-ICOS, anti-CD25, biotinylated lineage antibodies. Add 0.015 µg/106 cells of Brilliant Violet 421 Streptavidin.
- Incubate the tube at room temperature for 20 min.
- Wash the cells by adding 2 ml FACS buffer and centrifuge at 300 x g for 10 min.
- Proceed to FACS sorting. Gate and sort ILC2 as lineage negative, Sca-1+, ICOS+, KLRG1+ cells (see gating strategy in Figure 1).
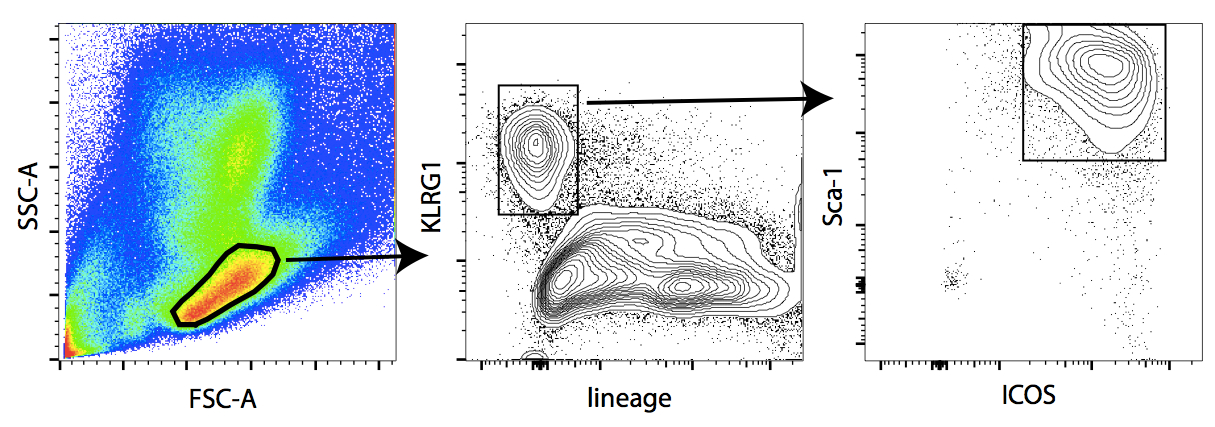
Figure 1. Gating strategy
Recipes
Note: All materials and reagents should be low endotoxin cell culture quality.
- Krebs-Ringer-Buffer (KRB)
154 mM NaCl
5.6 mM KCl
5.5 mM glucose
20.1 mM NaHCO3
Adjust pH to 7.4 with NaOH
Bring to final volume of 1,000 ml
Filter sterilize and store at 4 °C
- Collagenase IV solution
5,000 Digestion Units/ml in KRB buffer
Store stock solution at -20 °C
- DNase I solution
30,000 Units/ml in KRB buffer
Store stock solution at -20 °C
- 30% Biocoll
36.97 ml Biocoll 100%
63.03 ml PBS
Keep sterile at 4 °C
- 80% Biocoll
79.8 ml Biocoll 100%
20.1 ml PBS
Keep sterile at 4 °C
- FACS buffer
Add 5 ml fetal calf serum to 495 ml of PBS
- ACK buffer
4.145 g NH4Cl
0.5 g KHCO3
18.6 g EDTA
500 ml distilled water
Filter sterilize and keep at 4 °C
- PEB buffer
Phosphate buffered saline (PBS) containing 0.5 % BSA and 2 mM EDTA
Acknowledgments
This work was supported by the Collaborative Research Center 796 and the Priority program SPP1656 of the DFG (to S.W.) and the Interdisciplinary Center for Clinical Research (IZKF) of the University Medical Center Erlangen.
References
- Mchedlidze, T., Waldner, M., Zopf, S., Walker, J., Rankin, A. L., Schuchmann, M., Voehringer, D., McKenzie, A. N., Neurath, M. F., Pflanz, S. and Wirtz, S. (2013). Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 39(2): 357-371.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Mchedlidze, T. and Wirtz, S. (2014). Isolation of ILC2 from Mouse Liver. Bio-protocol 4(14): e1179. DOI: 10.21769/BioProtoc.1179.
Category
Immunology > Immune cell isolation > Lymphocyte
Cell Biology > Cell isolation and culture > Cell isolation
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











