- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Autoradiography of Pi Distribution in Barley Seedlings
Published: Vol 4, Iss 13, Jul 5, 2014 DOI: 10.21769/BioProtoc.1175 Views: 9409
Reviewed by: Ru Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualization of Nitric Oxide, Measurement of Nitrosothiols Content, Activity of NOS and NR in Wheat Seedlings
Sandeep B. Adavi [...] Shailendra K. Jha
Oct 20, 2019 6355 Views

A Quick Method to Quantify Iron in Arabidopsis Seedlings
Chandan Kumar Gautam [...] Wolfgang Schmidt
Mar 5, 2022 3934 Views

CAPS-Based SNP Genotyping for Nitrogen-Response Phenotypes in Maize Hybrids
Jannis Jacobs [...] Peter K. Lundquist
Dec 20, 2025 551 Views
Abstract
Phosphorus-32 and Phosphorus-33 are radioisotopes of phosphorus. These isotopes are used to trace ionic phosphorus and phosphorus compounds. This protocol is used to follow the movement of inorganic phosphate (PO43-) from a leaf tip to the rest of the plant.
Keywords: AutoradiogramMaterials and Reagents
- Barley seedlings
- Radioisotopes 32P or 33P labeled NaH2PO4 dissolved in water (MP Biomedicals, PerkinElmer or American Radiolabeled Chemicals)
- 5 mM CaSO4 solution
- Hydroponic culture solution (see Recipes)
Equipment
- Cling film
- 1.5 ml plastic tubes
- 15 ml plastic tubes (1.5 ml tube is fitted by opening a hole in the lid) (Figure 1)
- Cotton
- Plastic sponge
- Imaging plate (FCR Imaging Plate for general purpose) (Fujifilm Corporation) and plate cassette (FCR standard cassette) (Fujifilm Corporation)
- Imaging analyzer (GE Healthcare, model: Typhoon 9400 or other Radioisotope imaging analyzers)
Procedure
- Barley plants are germinated on moist filter paper for 2-3 days and then seedlings are grown in hydroponic culture for 7-8 days.
- Cotton is put in a 1.5 ml tube from which the cap has been removed, then a radioisotope medium consisting of 600 µl of 0.2 mM NaH232PO4 (specific activity 3.7 MBq nmol−1) in 5 mM CaSO4 is added. Cotton is put enough to absorb all 600 µl NaH232PO4, But, do not put too much cotton to keep it moisture.
- Into the 15 ml tube, an appropriate amount of incubation medium (5 mM CaSO4) is added. A barley plant sandwiched with sponge is put into the medium, and the barley leaf is manipulated into position against the plastic sponge separated from solution, such that when the smaller tube containing the cotton is mounted into a hole in the cap of the 15 ml tube, the tip of the leaf comes into contact with the cotton soaked in radioactive medium (Figure 1).
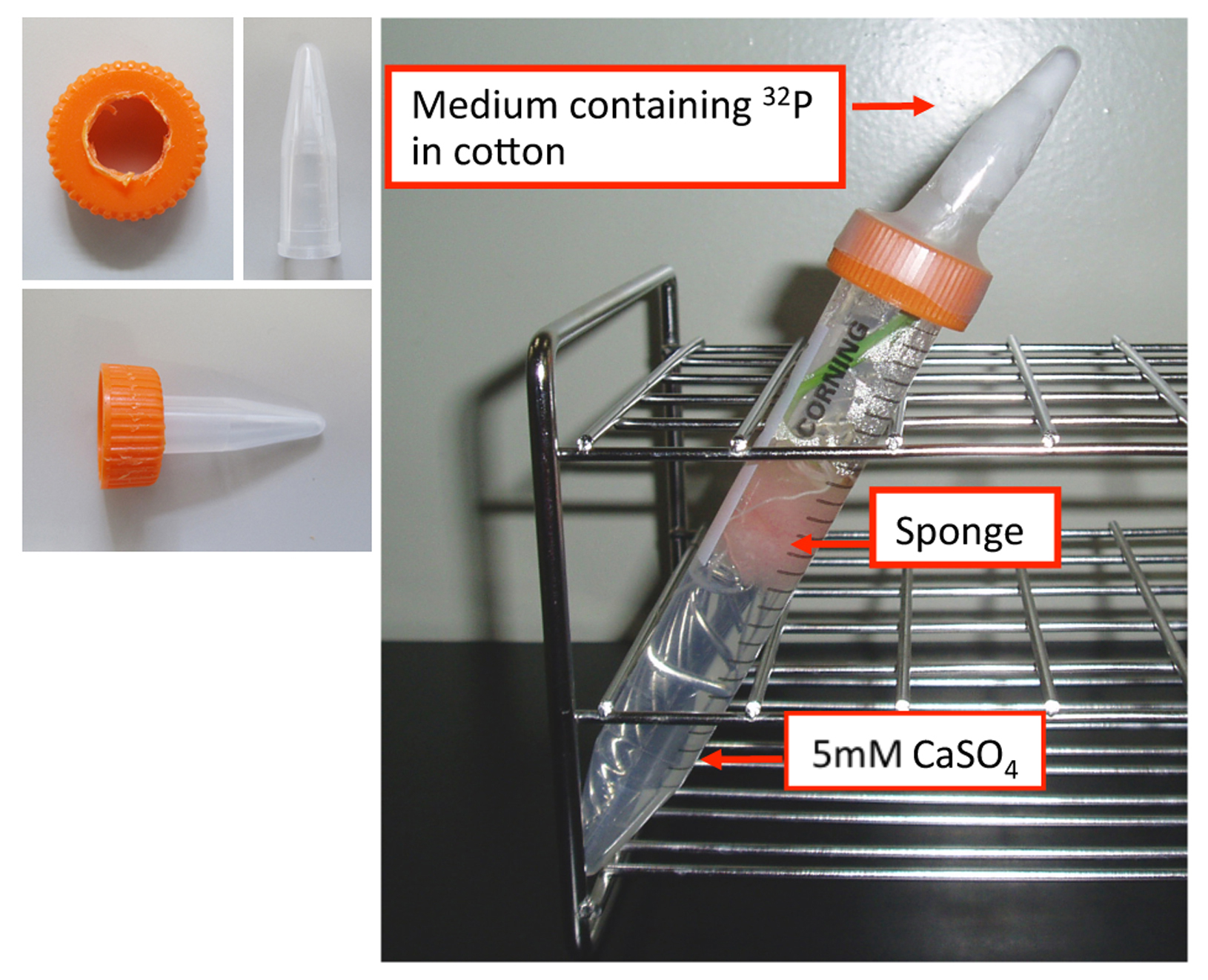
Figure 1. Setup for the radioisotopic labelling of a leaf tip
- After an appropriate labelling period (about 2 h) at 25 degrees, the sample is washed with water several times and wrapped with cling film. The sample is then set on an imaging plate and exposed (Figure 2).

Figure 2. Setup of the radiolabeled sample on an imaging plate
To avoid contamination of imaging plate with 32P, samples are wrapped by cling film. Wrapped sample is attached to the imaging plate by using binding case to expose.
- Examine the imaging plate with an imaging analyzer (Figure 3). 32P concentration is indicated by pseudo-color.
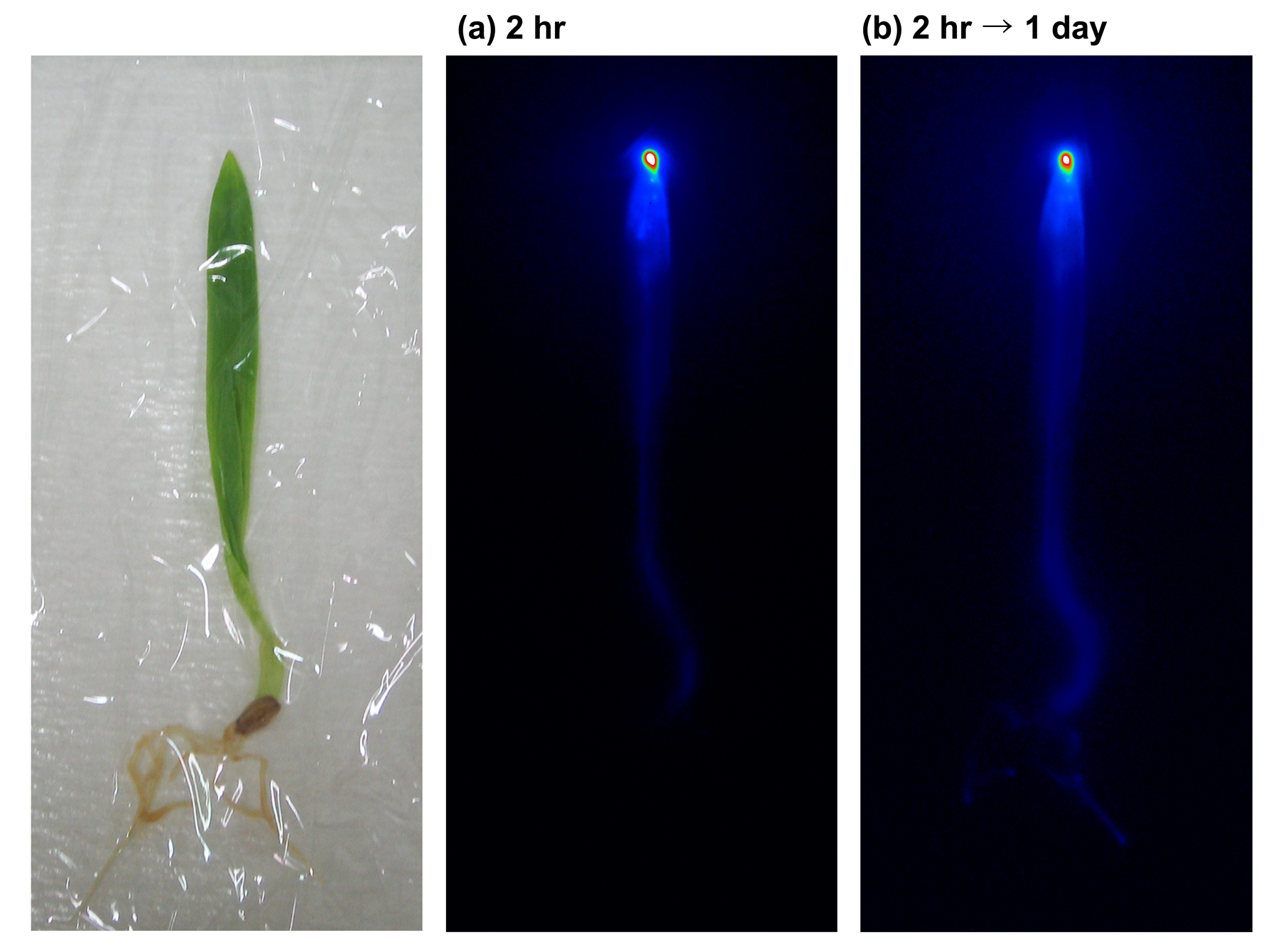
Figure 3. Autoradiogram of 32P distribution in a barley plant which was radiolabeled from the leaf tip
- Following imaging, plants can continue to be incubated in 5 mM CaSO4 solutions under the same light conditions for 1 or 2 d without cap in order to examine the movement of the radioisotope. In Figure 3 it can be seen that NaH232PO4 absorbed at the leaf tip was translocated to the root after one day of incubation.
Figure 4 shows autoradiograms taken every 2 days following the initial incubation of roots in a Pi-deficient barley plant with an appropriate amount of incubation medium mixed NaH232PO4 (Mimura et al., 1996)
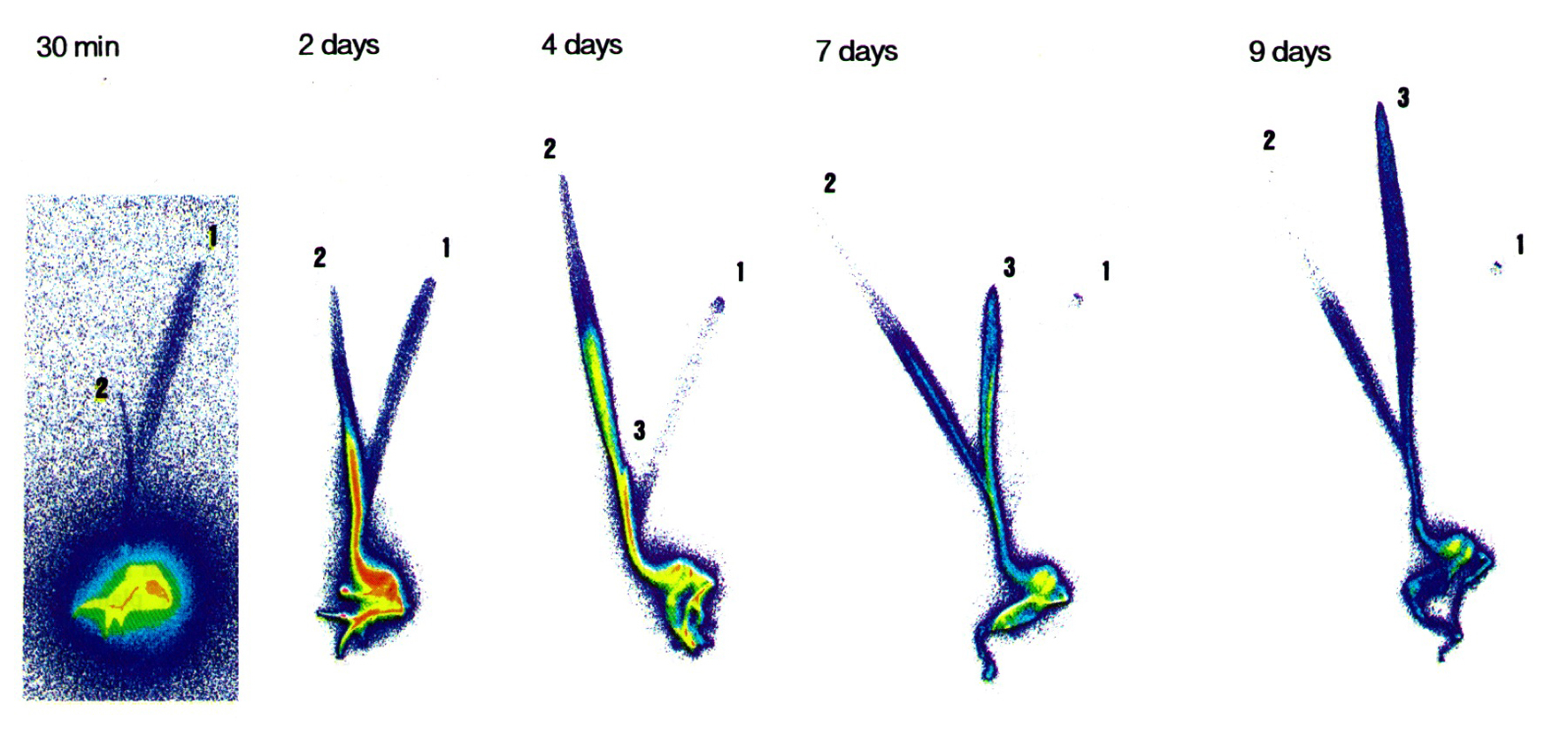
Figure 4. Autoradiogram of 32P distribution in 10 days old barley plant grown in a Pi-deficient medium, which was radiolabeled from the root. The number put at the leaves in order of development.
Notes
- Resolution and clarity of images are strongly dependent on the radioisotope contents of plants and exposure time on the imaging plate.
Recipes
- Hydroponic culture solution
9 mM KNO3
6 mM Ca (NO3)2
3 mM MgSO4
1.5 mM KH2PO4
0.125 mM Fe-EDTA
Micronutrients: 10 µM MnSO4, 1 µM CuSO4, 1 µM ZnSO4, 30 µM H3BO3, 30 µM (NH4)6Mo7O24, 0.1 µM CoCl2
Acknowledgments
This protocol was adapted from the following publications, Nagai et al. (2013) and Mimura et al. (1996). This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology and Japan Society for the Promotion of Science (JSPS), CREST of JST (Japan Science and Technology Corporation) and in part by Hyogo Science and Technology Association. The authors also thank Yokogawa Analytical Systems Inc. for use of ion chromatography equipment.
References
- Mimura, T., Sakano, K. and Shimmen, T. (1996). Studies on the distribution, re‐translocation and homeostasis of inorganic phosphate in barley leaves. Plant Cell Environ 19(3): 311-320.
- Nagai, M., Ohnishi, M., Uehara, T., Yamagami, M., Miura, E., Kamakura, M., Kitamura, A., Sakaguchi, S., Sakamoto, W., Shimmen, T., Fukaki, H., Reid, R. J., Furukawa, A. and Mimura, T. (2013). Ion gradients in xylem exudate and guttation fluid related to tissue ion levels along primary leaves of barley. Plant Cell Environ 36(10): 1826-1837.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kanno, S., Kurita, Y., Ohnishi, M. and Mimura, T. (2014). Autoradiography of Pi Distribution in Barley Seedlings. Bio-protocol 4(13): e1175. DOI: 10.21769/BioProtoc.1175.
Category
Plant Science > Plant physiology > Nutrition
Plant Science > Plant physiology > Ion analysis
Biochemistry > Other compound > Ion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link













