- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Bone Marrow Derived Eosinophil Cultures
Published: Vol 4, Iss 12, Jun 20, 2014 DOI: 10.21769/BioProtoc.1161 Views: 14163
Reviewed by: Fanglian HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3938 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 232 Views
Abstract
Eosinophils are multifunctional effector cells implicated in the pathogenesis of a variety of diseases including asthma, eosinophil gastrointestinal disorders and helminth infection. Mouse bone marrow derived progenitor cells can be differentiated into eosinophils following IL-5 exposure. These bone marrow derived eosinophils are fully differentiated at the end of a 14 day culture based on morphology and expression of molecular markers.
Materials and Reagents
- Mice
- Histopaque 1083 (Sigma-Aldrich, catalog number: 10831 -100ml)
- IMDM with Glutamax-I (Life Technologies, InvitrogenTM, catalog number: 31980-097 )
- Fetal Bovine Serum (FBS) (Atlanta Biologicals, catalog number: S11150 )
- Penicillin/Streptomycin (Life Technologies, InvitrogenTM, catalog number: 15140-122 )
- Stem Cell Factor (Pepro tech, catalog number: 250-03 )
- FLT-3 Ligand (Pepro tech, catalog number: 250-31L )
- Recombinant mouse IL-5 (Pepro tech, catalog number: 215-15 )
- PBS (Life Technologies, InvitrogenTM, catalog number: 14200-166 )
- Diff-Quick Stain Kit (Thermo Fisher Scientific, catalog numbers: 23-122-929 , 23-122-952 , and 23-122-937 )
- RBC lysis buffer (Sigma-Aldrich, catalog number: R7757 -100ml)
- CCR3 (R&D systems, Catalog number: FAB729F )
- Siglec-F (BD, PharmingenTM, Catalog number: 552126 )
- IMDM cell culture media (see Recipes)
Equipment
- 6-well tissue culture plate
- Dissection tools: scalpel, scissors
- 1 ml syringe
- 15 ml sterile centrifuge tube
- Centrifuge
- 37 °C, 5% CO2 cell culture incubator
- Microscope
- Hemocytometer
Procedure
- Collect femur/tibia from 6-8 mice.
Note: Mice between 6-8 weeks old will give the highest yield of low-density bone marrow fraction.
- Flush the bone marrow from femur/tibia with PBS, pipet up and down to break up the bone marrow into single cell suspension.
- Spin down the cells at 300 x g 8 min at 4 °C.
- Resuspend cells in 15 ml of RBC lysis buffer, incubate for 2 min at 37 °C.
Note: If less than 6-8 mice were used, there is no need to adjust the volume of RBC lysis buffer. If more than 6-8 mice were used, we recommend scaling up the RBC lysis buffer accordingly and also scaling up the amount of PBS used to neutralize the RBC lysis buffer in step 5.
- Add 30 ml of PBS to neutralize the RBC lysis buffer.
- Spin down the cells at 300 x g 8 min at 4 °C.
- Resuspend the cells in 16 ml of PBS.
- Add 4 ml of histopaque 1083 to each of 15 ml centrifuge tube.
- Carefully layer 4 ml of cells from step 7 to 4 ml of histopage 1083 prepared in step 8. Must use very slow speed to ensure that the layers are not disturbed. At the end of this step, there should be two layers: bottom clear layer contains histopage 1083, and top cloudy layer contaning cells.
- Centrifuge at 400 x g for exactly 30 min at room temperature. The brake on the centrifuge needs to be in the off position.
- Collect the low density fraction with a 1 ml micropipette. The low density fraction is at the interface between upper and lower layers. The approximate volume of this fraction is 0.5 ml to 1 ml.
Note: The low-density fraction is cloudy due to the low-density bone marrow cells at this fraction. The upper layer immediately above it is clear. The lower layer immediately below it should be significantly less cloudy compared to the low-density fraction.
- Wash the cells with 30 ml of PBS and spin down at 300 x g 8 min.
- Resusped the cells in IMDM with Glutamax-1 with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin supplemented with 100 ng/ml stem cell factor and 100 ng/ml FLT-3 ligand at a concentration of 1 x 106/ml.
- Plate 3 x 106 cells per well in 6 well plates (3 ml).
- Change culture media on day 2 by aspirating 1.5 ml of media from each well and adding 1.5 ml of new media (see Recipes).
Note: Be careful not to aspirate the cells as the cells are settled to the bottom of the plate but are not attached.
- On day 4 , collect all the cells, spin down the cells at 300 x g 8 min at 4 °C, wash once with 30 ml of PBS, then resuspend the cells at a concentration of 1 x 106/ml in IMDM with Glutamax-1 with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin supplemented with 10 ng/ml of IL-5.
- Change the culture culture media every other day. Cells should be counted and concentration adjusted to 1 x 106/ml during each media change.
- At day 14, collect mature eosinophils for further studies. Eosinophil maturity can be assessed by FACS staining for CCR3 and Siglec-F and/or Diff-Quik staining of cytospin preparations following the manufacturer’s protocol (Figure 1).
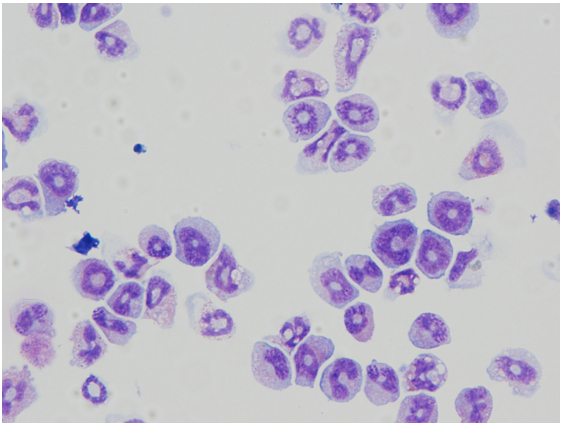
Figure 1. Morphology of mature bone marrow derived eosinophils. Morphology determined by Diff-Quik staining following the manufacturer’s protocol.
Recipes
- IMDM cell culture media
IMDM with Glutamax-1
10% FBS
100 U/ml penicillin
100 μg/ml streptomycin supplemented with 100 ng/ml stem cell factor
100 ng/ml FLT-3 ligand
Cellular concentration of 1 x 106/ml
Acknowledgments
This protocol has been adapted from Lu et al. (2013).
References
- Lu, T. X., Lim, E. J., Besse, J. A., Itskovich, S., Plassard, A. J., Fulkerson, P. C., Aronow, B. J. and Rothenberg, M. E. (2013). MiR-223 deficiency increases eosinophil progenitor proliferation. J Immunol 190(4):1576-1582.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lu, T. X. and Rothenberg, M. E. (2014). Bone Marrow Derived Eosinophil Cultures. Bio-protocol 4(12): e1161. DOI: 10.21769/BioProtoc.1161.
Category
Immunology > Immune cell isolation > Maintenance and differentiation
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










