- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
EML Erythroid and Neutrophil Differentiation Protocols
Published: Vol 4, Iss 12, Jun 20, 2014 DOI: 10.21769/BioProtoc.1151 Views: 12415
Reviewed by: Lin FangFanglian HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3951 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2419 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 246 Views
Abstract
Erythroid-Myeloid-Lymphoid cells (EML) are a multipotent haematopoietic cell line of mouse bone marrow origin capable of long-term maintenance in vitro in the presence of SCF (stem cell factor) (Tsai et al., 1994). The self-renewal capacity of the EML cell line is conferred by the presence of a dominant-negative retinoic acid receptor (RAR) originally delivered by retroviral transduction (Tsai et al., 1994), which arrests cells at an early progenitor stage blocked from normal progression into myeloid differentiation. The presence of the RAR trans-gene does not interfere with erythroid differentiation, and it is possible to capture a low percentage of early erythroid, but not myeloid, committed cells in maintenance cultures (Pina et al., 2012; Ye et al., 2005).
Cells can be driven into granulocytic/neutrophil differentiation through the use of high doses of retinoic acid (RA), which overcomes the differentiation block. It should be noted that these pharmacological doses of RA are not compatible with erythroid differentiation, and it is hence not viable to obtain robust erythroid and myeloid differentiation in the same assay. Indeed, colonies scored as mixed-lineage in CFC assays are a mixture of undifferentiated and erythroid cells (Tsai et al., 1994). Nevertheless, robust single-lineage erythroid and neutrophil differentiation can be obtained in liquid culture under defined cytokine conditions, as specified below.
Materials and Reagents
- IMDM (powder or liquid)
- Horse serum (HS) (may require batch testing if low cell viability)
- Foetal bovine serum (FBS)
- L-Glutamine
- Penicillin/Streptomycin (P/S)
- SCF-conditioned medium (SCF-CM) (see Notes)
- Recombinant human erythropoietin (e.g. epoietin alpha, Amgen) (obtained through hospital pharmacy under appropriate local guidelines)
- Recombinant mouse interleukin-3 (IL-3) (e.g. Pepro Tech, catalog number: 213-13 )
- Recombinant mouse granulocyte-monocyte colony-stimulating factor (GM-CSF) (e.g. Pepro Tech, catalog number: 315-03 )
- All-Trans Retinoic Acid (ATRA) (e.g. Sigma-Aldrich, catalog number: 302-79-4 , reconstitute in ethanol)
- Culture supplements and antibiotics (L-Glutamine and Penicillin/Streptomycin)
- Monoclonal anti-mouse antibodies for flow cytometry
- C-kit/CD117 (clone 2B8) (e.g. PE-Cy7, eBioscience, catalog number: 25-1171 ) (suggested use at 1:100 dilution)
- CD34 (clone RAM34) (e.g. Alexa-Fluor 647, eBioscience, catalog number: 51-0341 ) (suggested use at 1:100 dilution)
- Mac-1/CD11b (clone M1/70) (e.g. PE, eBioscience, catalog number: 12-0112 ) (suggested use at 1:100 dilution)
- Gr1/Ly6C (clone RB6-8C5) (e.g. FITC, eBioscience, catalog number: 11-5931 ) (suggested use at 1:100 dilution)
- Sca-1/Ly6A/E (clone D7) (e.g. Pacific blue, BioLegend, catalog number: 122520 ) (suggested use at 1:50 dilution)
Note: Stain on ice, in culture medium, for 20 min; wash with 10-20x volume of medium; pellet cells at 400 x g for 5 min. Re-suspend in 300-500 μl of medium for FACS analysis.
- C-kit/CD117 (clone 2B8) (e.g. PE-Cy7, eBioscience, catalog number: 25-1171 ) (suggested use at 1:100 dilution)
- Trypan blue
Equipment
- T175 tissue culture flasks with filtered cap (for production of conditioned medium)
- T75 or T25 tissue culture flasks with filtered cap, or 6-well plates
Note: For EML cultures, the size of the tissue culture vial is determined by the cell number seeded at the cell densities indicated in the protocol.
- Cell culture incubator
Procedure
- Maintenance culture conditions
- EML cells maintenance culture conditions are IMDM with 5% HS, 2 mM L-glutamine and 1x penicillin/streptomycin (P/S) and 10-15% of SCF-conditioned medium (SCF-CM) obtained from BHK-MKL cells.
Note: The original cultivars of EML cells were grown in medium supplemented with rat SCF at a final concentration of 200 ng/ml (Tsai et al., 1994).
- Cells should be seeded at 2 x 104 cells/ml and split to the initial dilution when a cell density of 105-2 x 105/ml (absolute maximum) is reached. This typically means splitting the cultures every 2 or sometimes 3 days. Cultures die rapidly at higher cell densities.
Note: The dead cell fraction in maintenance cultures, as judged by Trypan blue exclusion, should not exceed 5-8% (10% is acceptable if not indicative of a culture exhaustion trend.).
- Cultures can be maintained for at least 20 passages post-thaw without any significant changes in biology.
- EML cells maintenance culture conditions are IMDM with 5% HS, 2 mM L-glutamine and 1x penicillin/streptomycin (P/S) and 10-15% of SCF-conditioned medium (SCF-CM) obtained from BHK-MKL cells.
- Erythroid differentiation conditions
- Use EML cells in logarithmic growth phase from a maintenance culture, and plate them at a seeding concentration of 2 x 104 cells/ml in fresh maintenance culture medium + recombinant human erythropoietin (EPO) at a final concentration of 10 U/ml.
- EML erythroid differentiation cultures are very sensitive to cell crowding, so opt for increasing culture volumes rather than initial cell densities if high numbers of cells are required.
- Culture for 2 days. After this initial period, wash cells 2-3x in IMDM with 5% HS + 2 mM L-glutamine and 1x P/S, and re-seed at 2 x 104 cells/ml in IMDM, 5% HS, 2 mM L-glutamine and 1x P/S, with 2.5% SCF-CM and 10 U/ml EPO.
- Differentiate cells for a total of 7 days (so, 5 additional days), with fresh EPO (10 U/ml) added on day 5 to the existing culture.
- It is expected to observe an average 20-fold expansion in cell numbers during the culture period, with cell viability decreasing gradually as cells differentiate, to a minimum of 40-50% at day 7.
- The extent of cell differentiation can be determined by flow cytometry staining with C-Kit, Sca1 and CD34, with accumulation of differentiated Sca1loCD34-C-kitlo/- cells (referred to as Kit-, see Figure 1 for representative plot) (Pina et al., 2012). It is expected that 20-30% of the culture become Kit- by day 6 of differentiation.
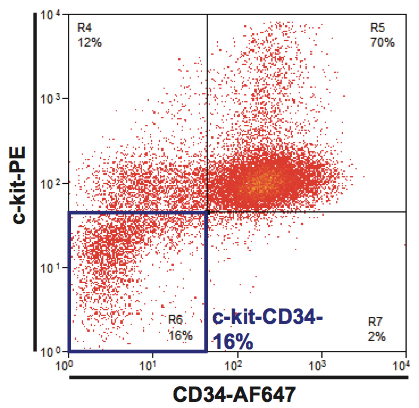
Figure 1. Erythroid differentiation of EML cells. A representative plot of flow cytometry analysis at day 4 is shown; c-kit-CD34- cells are Sca1lo/-. In this instance, cultures were seeded at day 0 with a population of cells with culture-reconstituting (or self-renewal) potential (Pina et al., 2012).
- When erythroid differentiation cultures are seeded with committed erythroid cells (e.g. sorted Sca1loCD34- cells cultured as per B1 above) (Pina et al., 2012), 60-80% of the culture are Kit- by day 4 of differentiation, reflecting faster differentiation kinetics from committed cells.
- Not unexpectedly, cultures seeded with erythroid-committed cells are less proliferative, with a 10-fold expansion in cell numbers expected by day 4, after which the cultures plateau and become less viable.
- Use EML cells in logarithmic growth phase from a maintenance culture, and plate them at a seeding concentration of 2 x 104 cells/ml in fresh maintenance culture medium + recombinant human erythropoietin (EPO) at a final concentration of 10 U/ml.
- Neutrophil differentiation conditions
- Use EML cells in logarithmic growth phase from a maintenance culture, and plate them at a seeding concentration of 2 x 104 cells/ml in fresh maintenance culture medium + 10 ng/ml of recombinant mouse IL-3 and 10 μM ATRA.
- Viability is less affected by cell crowding than in erythroid differentiation, and it is thus possible to seed cultures at higher densities (0.5-1 x 105) if high cell numbers are required.
- After 2 days, wash cells 2-3x in IMDM with 5% HS + 2 mM L-glutamine and 1x P/S, and re-seed at 2 x 104 cells/ml in IMDM, 5% HS, 2 mM L-glutamine and 1x P/S, with 2.5% SCF-CM + 10 ng/ml of recombinant mouse GM-CSF and 10 μM ATRA.
- Keep the culture growing for a total of 7 days (so, 5 additional days), with fresh GM-CSF (10 ng/ml) added on day 5 to the existing culture.
- It is expected to observe an average 10-fold expansion in cell numbers during the culture period, with viabilities close to 80% or higher throughout the duration of the culture.
- The extent of cell differentiation can be determined by flow cytometry after staining with Gr-1 and Mac-1 antibodies (Pina et al., 2012), with accumulation of granulocytic Gr1+Mac-1+ cells to an expected proportion of 40-50% by days 4-5 of culture (see Figure 2 for representative plot). Proportions can drop thereafter, although absolute numbers of differentiated cells continue to increase.
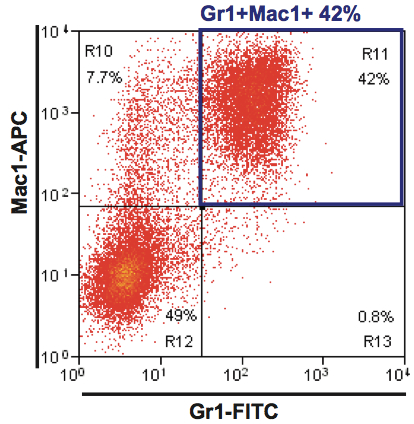
Figure 2. Neutrophil differentiation of EML cells. A representative plot of flow cytometry analysis at day 6 is shown.
- Use EML cells in logarithmic growth phase from a maintenance culture, and plate them at a seeding concentration of 2 x 104 cells/ml in fresh maintenance culture medium + 10 ng/ml of recombinant mouse IL-3 and 10 μM ATRA.
Notes
- SCF-conditioned medium (SCF-CM)
- One possible source are BHK cells expressing the murine kit ligand protein (MKL, or SCF).
- Conditioned medium can be produced in T175 flasks with a total production volume of 60 ml/flask.
- Culture the cells in IMDM with 10% FBS, 2 mM L-Glutamine and 1x Penicillin/Streptomycin until they reach 80% confluence.
- At this stage replace with fresh medium and culture for an additional 48 h, at which point the supernatant is collected, floating cells removed by pelletting at 400 x g, 5 min, and the medium filtered through a 0.2 μm mesh.
- Conditioned medium can be aliquoted and stored for several months at -20 °C without loss of activity.
- One possible source are BHK cells expressing the murine kit ligand protein (MKL, or SCF).
Acknowledgments
The protocol herein described was originally reported in Pina et al. (2012). Its development and implementation was financially supported by the Medical Research Council of the United Kingdom, Leukaemia and Lymphoma Research, EuroSyStem and STEMEXPAND.
References
- Orford, K., Kharchenko, P., Lai, W., Dao, M. C., Worhunsky, D. J., Ferro, A., Janzen, V., Park, P. J. and Scadden, D. T. (2008). Differential H3K4 methylation identifies developmentally poised hematopoietic genes. Dev Cell 14(5): 798-809.
- Pina, C., Fugazza, C., Tipping, A. J., Brown, J., Soneji, S., Teles, J., Peterson, C. and Enver, T. (2012). Inferring rules of lineage commitment in haematopoiesis. Nat Cell Biol 14(3): 287-294.
- Tsai, S., Bartelmez, S., Sitnicka, E. and Collins, S. (1994). Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev 8(23): 2831-2841.
- Ye, Z. J., Kluger, Y., Lian, Z. and Weissman, S. M. (2005). Two types of precursor cells in a multipotential hematopoietic cell line. Proc Natl Acad Sci U S A 102(51): 18461-18466.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Pina, C., Fugazza, C. and Enver, T. (2014). EML Erythroid and Neutrophil Differentiation Protocols. Bio-protocol 4(12): e1151. DOI: 10.21769/BioProtoc.1151.
Category
Cell Biology > Cell isolation and culture > Cell differentiation
Stem Cell > Adult stem cell > Hematopoietic stem cell
Stem Cell > Adult stem cell > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









