- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Protoplast Preparation and Determination of Cell Death
Published: Vol 4, Iss 12, Jun 20, 2014 DOI: 10.21769/BioProtoc.1149 Views: 19046
Reviewed by: Ru Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Acidified Blue Ink-staining Procedure for the Observation of Fungal Structures Inside Roots of Two Disparate Plant Lineages
Jill Kowal [...] Sophie Lane
Oct 20, 2020 5045 Views

Transgene-free Genome Editing in Grapevine
Edoardo Bertini [...] Sara Zenoni
Feb 20, 2025 2526 Views

Isolation and Transfection of Protoplasts From Maize Mesophyll Cells
Lauren A. Higa [...] Zhi-Yan Du
Feb 5, 2026 100 Views
Abstract
The protoplasts assay constitutes a powerful tool that allows an easy uptake of active agents and a precise quantification of cell death induction in different populations. Our study showed that the basal level of cell death in our controls is low and stable throughout the length of our experiments (Danon et al., 2005; Pineau et al., 2013). In addition, the data obtained from the protoplast assay are applicable to intact seedlings, where it is possible to see differences in the intensity of necrotic lesions (Danon et al., 2006) even if those differences are not as easily and clearly quantifiable as with the protoplast assay.
Keywords: ProtoplastMaterials and Reagents
- Plant materials: Arabidopsis thaliana sterile seedlings grown in vitro from 1 to 3 weeks in the required conditions
- Gamborg B5 medium salt and vitamins (Duchefa Biochemie, catalog number: G0210 )
- 2-(N-Morpholino) ethanesulfonic acid (MES)
- Mannitol
- Digestion enzymes
- Culture medium (see Recipes)
- Digestion medium (see Recipes)
- W5A medium (see Recipes)
- 21% sucrose (see Recipes)
- 1% Evans blue (see Recipes)
Equipment
- Light microscope
- An appropriate slide (e.g. Malassez slide)
- Centrifuge for 15 ml and 50 ml Falcon tubes
- Sterile pipette
- Filter (0.45 µm)
- Hemocytometer (VWR International, catalog number: 631-0975 )
- Cell dissociation sieve (100 µm) (Sigma-Aldrich, catalog number: CD1-1KT ) sieve mounted on a sterile 150 ml beaker
- Micropore tape (VWR International, catalog number: 115-8172 )
Procedure
- Protoplast extraction
- Harvest whole young seedlings with sterile forceps and put them in a sterile Petri plate.
- Cover the leaves with the digestion solution (~15 ml for a 10 cm round plate).
- Close the plates with micropore tape and let digest at room temperature in the dark, overnight (~16 h).
- Separate the protoplasts from the seedlings by pipetting and releasing delicately the medium using a 3 ml sterile pipette.
- Filter the medium containing the protoplasts with a sieve of 100 µm, mounted on a sterile 150 ml beaker.
- Transfer the content of the beaker in a sterile 15 ml tube.
- Centrifuge 5 min at 180 x g at room temperature, eliminate the supernatant and resuspend the pellet in 4 ml of W5A medium.
- Very slowly deposit the protoplasts on 8 ml of 21% sucrose using a 3 ml sterile pipette (use a 50 ml tube if you have a lot of material, and a 15 ml tube otherwise).
- Centrifuge 13 min, 720 x g at room temperature.
- Carefully harvest the living protoplast at the surface of the sucrose solution (thin cushion, Figure 1) using a 3 ml sterile pipette and transfer them into a 15 ml sterile tube.
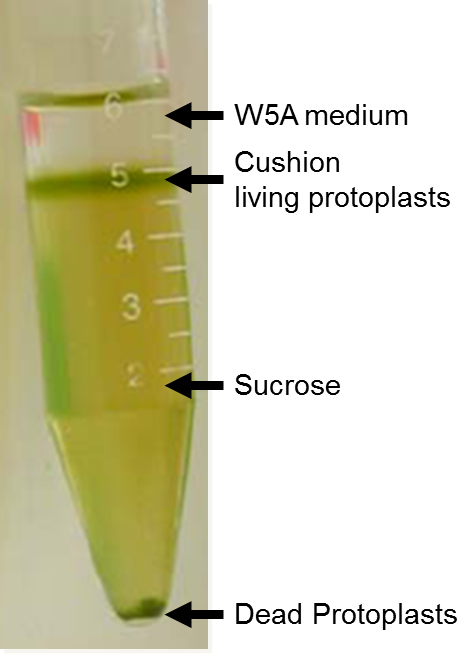
Figure 1. Living and dead protoplasts after centrifugation on 21% sucrose - Complete to 15 ml with W5A and calculate protoplast concentration on an aliquot using an appropriate slide (e.g. Malassez slide) and a light microscope (20x magnification).
- Centrifuge 5 min, at 180 x g at room temperature.
- Eliminate the supernatant, and resuspend the pellet in the appropriate volume of culture medium depending on the required concentration.
- Harvest whole young seedlings with sterile forceps and put them in a sterile Petri plate.
- Cell death determination
- Add 1 µl of 1% Evans blue to an aliquot of 25 µl of protoplast.
- Using light microscopy and an appropriate slide (e.g. Malassez slide), calculate the percentage of dead protoplasts that are stained with Evans Blue (blue protoplast/total protoplast, Figure 2).

Figure 2. Control protoplast (left panel) and singlet oxygen-treated protoplast. Dark blue protoplasts are dead (Danon et al., 2005).
- Add 1 µl of 1% Evans blue to an aliquot of 25 µl of protoplast.
Recipes
- Culture medium (500 ml)
Gamborg B5 medium salt and vitamins: 3.2 g
0.4 M mannitol: 36.4 g
0.4 M glucose: 36 g
0.1% MES: 0.5 g
Complete to 500 ml with distilled H2O
Go to pH 5.7 with KOH
Sterilize by autoclave and keep at 4 °C - Digestion medium (500 ml)
0.4 M mannitol: 36.4 g
1% cellulase: 5 g
0.25% macerozyme: 1.25 g
8 mM CaCl2: 0.44 g
0.1% MES: 0.5 g
Complete to 500 ml with distilled H2O
Homogenise until complete dissolution
Go to pH 5.7 with KOH
Sterilize by 0.45 µm filtration (it is easier to centrifuge before 5 min at 16,000 x g)
Aliquot 10 ml and keep at -20 °C - W5A medium (2,000 ml)
5 mM glucose: 1.8 g
154 mM Nacl: 18 g
125 mM CaCl2: 27.8 g
5 mM KCl: 0.76 g
0.1% MES: 2 g
Complete to 2,000 ml with distilled H2O
Adjust to pH 5.7 with KOH
Sterilize by autoclave and keep at RT - 21% sucrose (100 ml)
Sucrose: 21 g
Complete to 100 ml with distilled H2O
Sterilize by autoclave and keep at RT - 1% Evans blue (100 ml)
Dissolve 1 g Evans blue in 100 ml water
Acknowledgments
This work was supported by Sorbonne Universités, UPMC Univ Paris 06 and the Centre National de la Recherche Scientifique.
References
- Danon, A., Coll, N. S. and Apel, K. (2006). Cryptochrome-1-dependent execution of programmed cell death induced by singlet oxygen in Arabidopsis thaliana. Proc Natl Acad Sci U S A 103(45): 17036-17041.
- Danon, A., Miersch, O., Felix, G., Camp, R. G. and Apel, K. (2005). Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J 41(1): 68-80.
- Pineau, B., Bourge, M., Marion, J., Mauve, C., Gilard, F., Maneta-Peyret, L., Moreau, P., Satiat-Jeunemaitre, B., Brown, S. C., De Paepe, R. and Danon, A. (2013). The importance of cardiolipin synthase for mitochondrial ultrastructure, respiratory function, plant development, and stress responses in Arabidopsis. Plant Cell 25(10): 4195-4208.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Danon, A. (2014). Protoplast Preparation and Determination of Cell Death. Bio-protocol 4(12): e1149. DOI: 10.21769/BioProtoc.1149.
Category
Plant Science > Plant cell biology > Cell isolation
Cell Biology > Cell viability > Cell death
Cell Biology > Cell staining > Whole cell
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










