- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Purification and Structural Analysis of QS-inhibiting Compounds from Staphylococcus delphini
Published: Vol 4, Iss 11, Jun 5, 2014 DOI: 10.21769/BioProtoc.1146 Views: 8445
Reviewed by: Fanglian HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

β-lactamase (Bla) Reporter-based System to Study Flagellar Type 3 Secretion in Salmonella
Fabienne F. V. Chevance and Kelly T. Hughes
Jun 20, 2023 1775 Views

Determination of Poly(3-hydroxybutyrate) Content in Cyanobacterium Synechocystis sp. PCC 6803 Using Acid Hydrolysis Followed by High-performance Liquid Chromatography
Janine Kaewbai-ngam [...] Tanakarn Monshupanee
Aug 20, 2023 1825 Views

An HPLC-based Assay to Study the Activity of Cyclic Diadenosine Monophosphate (C-di-AMP) Synthase DisA from Mycobacterium smegmatis
Avisek Mahapa [...] Dipankar Chatterji
Dec 20, 2024 1790 Views
Abstract
The knowledge that many pathogens rely on cell-to-cell communication mechanisms known as quorum sensing, opens a new disease control strategy: quorum quenching. Here we present a purification protocol for molecules excreted by a group of Gram-positive zoonotic pathogen bacteria, the ‘Staphylococcus intermedius group’, that suppress the quorum sensing signaling and inhibit the growth of a broad spectrum of Gram-negative beta- and gamma-proteobacteria. These compounds were isolated from Staphylococcus delphini (S. delphini). They represent a new class of quorum quenchers with the chemical formula N-[2-(1H-indol-3-yl)ethyl]-urea and N-(2-phenethyl)-urea, which we named yayurea A and B, respectively. These substances can be isolated and purified from the culture supernatant using this upscalable purification method.
Materials and Reagents
- Staphylococcus delphini DSMZ20771 strain (DSM number: 20071 )
Note: DSMZ stands for Deutsche Sammlung von Mikroorganismen und Zellkulturen. - Tryptic Soy Broth (TSB) (Sigma-Aldrich, catalog number: T8907 )
- Amberilite XAD-16 resin (Sigma-Aldrich, catalog number: 1-0379 )
- Methanol
- Acetic acid (Merck KGaA)
- Amberilite IRC 50 cation exchange resin (SERVA Electrophoresis GmbH, catalog number: 40501 )
- Sodium hydroxide
- Ethanol
- 50 mM and 1 M sodium phosphate buffer
- SP sepharose cation exchange column (GE Healthcare, catalog number: 17-5161-01 )
- Sodium chloride
- Trifluoroacetic acid (TFA) (Sigma-Aldrich, catalog number: T6508 )
- Phosphoric acid for HPLC (Sigma-Aldrich, catalog number: 79606 )
- Acetonitrile for HPLC (Mallinckrodt Baker, catalog number: 9012 )
Equipment
- 37 °C shaking incubator (Infors AG)
- Centrifuge (Eppendorf)
- Rotary evaporator (BÜCHI Labortechnik AG)
- Äkta FPLC equipped with P-900, UV-900, PH/C-900 (GE Healthcare)
- Preparative HPLC System equipped with Bischoff HPLC compact pump QC-P 2250 and Multiwavelength detector QC-1157 (Bischoff)
- Nucleosil 100 C-18 (8 x 250 mm column) (MACHEREY-NAGEL, catalog number: 715332.80 )
- Agilent 1200 series HPLC system (Agilent)
- Waters XBridge C18 (5 mm, 4.6 x 150 mm column) (Waters, part number: 186003116 )
Procedure
- S. delphini is cultivated in 100 ml TSB at 37 °C on a shaking incubator at 150 rpm for 20 h.
- Cells are centrifuged at 5,000 rpm (4,500 x g) at 4 °C for 10 min and the supernatant is applied on to a column filled with 10 ml Amberilite XAD-16 resin.
- The column is first washed with 5 bed volumes each of milliQ water, then with 40% and 60% methanol and finally eluted with 80% methanol containing 5% acetic acid at a flow rate of 10 bed volumes per hour.
- The eluate is evaporated using a rotary evaporator until all methanol is removed.
- The eluate is resuspended with 50 ml water and the pH adjusted to 7.0 with 1 M NaOH. It is then applied on to a column filled with 10 ml Amberilite IRC-50 cation exchange resin.
- The column is washed first with water, then with 70% ethanol and eluted with 80% ethanol acidified with 5% acetic acid each with 5 bed volumes at a flow rate of 10 bed volumes per hour.
- The eluate is concentrated using a rotary evaporator until all methanol is removed.
- The eluate is diluted with water and adjusted to a final concentration of 50 mM sodium phosphate using 1 M sodium phosphate buffer and pH is lowered to 4.2 using 85% phosphoric acid.
- In the third purification step, the eluate is separated on a 5 ml SP sepharose cation exchange column with a linear 0 to 1 M NaCl gradient in 50 mM sodium phosphate buffer on an Äkta purifier FPLC at a flow rate of 3 ml/min.
- The final purification and desalting of each peak is carried out by reversed phase preparative HPLC (RP-PHPLC) on a nucleosil 100 C-18, 8 x 250 mm column with a linear water acetonitrile (containing 0.1% TFA) gradient of 0% to 60% in 25 min.
- Purified compounds are lyophilized and stored at -20 °C.
- Qualitative analysis is carried out on an Agilent 1200 HPLC system and a RP-HPLC Waters xBridge C18, 5 mm, 4.6 x 150 mm column. Compounds are eluted with a 15 min linear gradient of aquaeus phosphoric acid (0.1% vol/vol) to acetonitrile at a flow rate of 1.5 ml/min and detected at 210 nm.
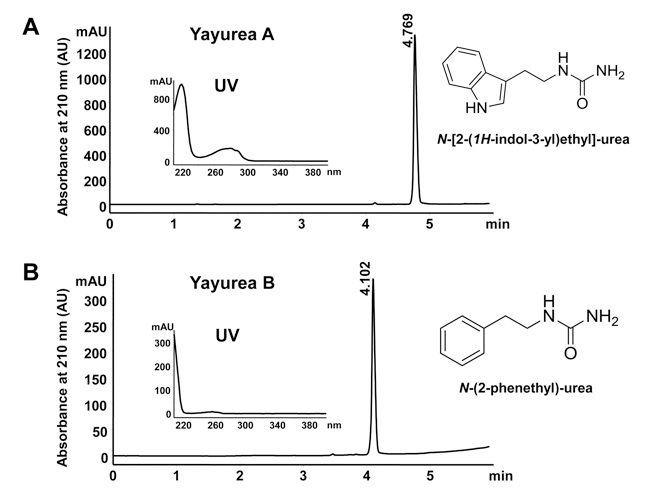
Figure 1. RP-HPLC profile, UV-spectrum and structures of the two QS-inhibitors purified from S. delphini. A. QS-inhibitor, N-[2-(1H-indol-3-yl)ethyl]-urea (yayurea A). B. QS-inhibitor, N-(2-phenethyl)-urea (yayurea B). RP-HPLC is carried out on an Agilent 1200 and Waters XBridge C18, 5 mm, 4.6 x 150 mm column; compounds are eluted with a 15 min linear gradient of 0.1% phosphoric acid to acetonitrile at a flow rate of 1.5 ml/min.
Acknowledgments
This work was funded by grants from the Landesgraduiertenförderung Baden Württemberg “Antimicrobial compounds” and the Deutsche Forschungsgemeinschaft SFB 766.This protocol was adapted from the original published paper Chu et al. (2013).
References
- Chu, Y. Y., Nega, M., Wölfle, M., Plener, L., Grond, S., Jung, K. and Götz, F. (2013). A new class of quorum quenching molecules from Staphylococcus species affects communication and growth of gram-negative bacteria. PLoS Pathog 9(9): e1003654.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chu, Y., Nega, M. and Götz, F. (2014). Purification and Structural Analysis of QS-inhibiting Compounds from Staphylococcus delphini. Bio-protocol 4(11): e1146. DOI: 10.21769/BioProtoc.1146.
- Chu, Y. Y., Nega, M., Wölfle, M., Plener, L., Grond, S., Jung, K. and Götz, F. (2013). A new class of quorum quenching molecules from Staphylococcus species affects communication and growth of gram-negative bacteria. PLoS Pathog 9(9): e1003654.
Category
Microbiology > Microbial signaling > Quorum sensing
Microbiology > Microbial biochemistry > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









