Improve Research Reproducibility A Bio-protocol resource
- Protocols
- Articles and Issues
- About
- Become a Reviewer
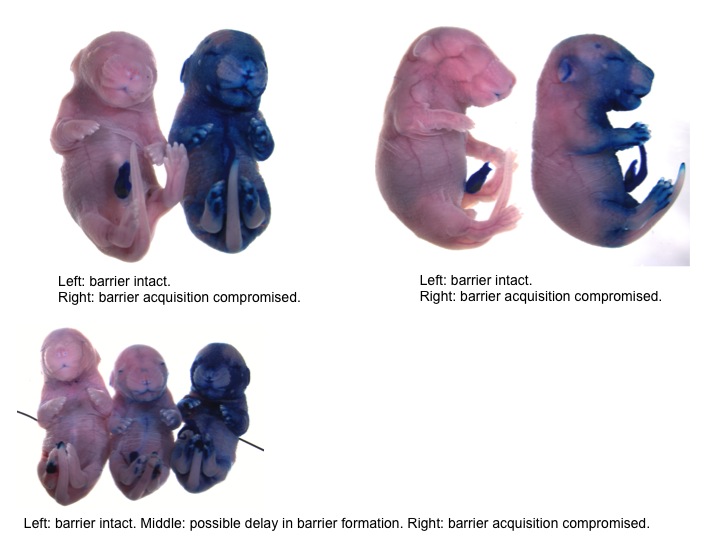
Barrier Function Assay
Published: Vol 4, Iss 10, May 20, 2014 DOI: 10.21769/BioProtoc.1133 Views: 11141
Reviewed by: Lin FangFanglian HeAnonymous reviewer(s)
How to cite
Favorite
Cited by