- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immunostaining Protocol: P-Stat3 (Xenograft and Mice)
Published: Vol 4, Iss 9, May 5, 2014 DOI: 10.21769/BioProtoc.1120 Views: 11048
Reviewed by: Lin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Analysis of the Ubiquitination and Phosphorylation of Vangl Proteins
Di Feng [...] Bo Gao
Oct 20, 2022 3424 Views

Isoform-specific, Semi-quantitative Determination of Highly Homologous Protein Levels via CRISPR-Cas9-mediated HiBiT Tagging
Kristina Seiler [...] Mario P. Tschan
Jul 20, 2023 2521 Views

Time-Lapse Into Immunofluorescence Imaging Using a Gridded Dish
Nick Lang [...] Andrew D. Stephens
Feb 20, 2026 55 Views
Abstract
We sought to understand the mechanisms behind the potent effect of stromal TGF-beta program on the capacity of colorectal cancer (CRC) cells to initiate metastasis. We discovered that mice subcutaneous tumors and metastases generated in the context of a TGF-beta activated microenvironment displayed prominent accumulation of p-STAT3 in CRC cells compared with those derived from control cells. STAT3 signaling depended on GP130 as shown by strong reduction of epithelial p STAT3 levels upon GP130 shRNA-mediated knockdown in CRC cells.
Materials and Reagents
- Paraffin sections (subcutaneous tumors samples or liver metastasis from nude mice respectively injected subcutaneously or intrasplenic with CRC cells)
- XILOL
Note: Xylol also referred to as xylene or dimethylbenzene is a solvent used in histology as a clearing agent to remove paraffin from dried microscope slides prior to staining.
- MilliQ H2O
- Wash buffer (Dako, catalog number: K800721 )
- Rabbit anti-P-Stat3 (Cell Signaling Technology, catalog number: 9145S )
- BrightVision poly-HRP anti- Rabbit (Immunologic, catalog number: DPVR110HRP )
- Envision FLEX antibody diluent (Dako, catalog number: K8006 )
- Peroxidase Blocking Solution (Dako, catalog number: S202386 )
- ImmPACT DAB (Vector Laboratories, catalog number: SK-4105 )
- DPX mounting media (Sigma-Aldrich, catalog number: 06522 )
- Hematoxylin
- Tris/EDTA (pH 9.0) (see Recipes)
Equipment
- Oven
- Immunostaining apparatus
Procedure
- Stove samples at 65 °C just before starting the immunostaining technique. Remove the samples from the oven when the wax present in sections is completely undone.
- De-waxing and rehydration: Place slides in a rack to perform following washes (bath).
- XILOL: 10 min
- XILOL: 10 min
- XILOL: 5 min
- 100% EtOH: 10 min
- 100% EtOH: 5 min
- 96% EtOH: 5 min
- 90% EtOH: 10-15 times
- 80% EtOH: 10-15 times
- 70% EtOH: 10-15 times
- 50% EtOH: 10-15 times
- 25% EtOH: 10-15 times
- H2O MilliQ: 10-15 times
- XILOL: 10 min
- Antigen retrieval.
- Tris-EDTA Buffer (pH 9.0)
- Time: 20 min in boiling water
- Tris-EDTA Buffer (pH 9.0)
- 3 washes 5 min with 1 ml 1x wash buffer.
- Blocking endogenous peroxidase.
- 200 ml Peroxidase Blocking Solution
- Time: 10 min
- 200 ml Peroxidase Blocking Solution
- 3 washes 5 min with 1 ml 1x wash buffer.
- Incubation with primary antibody.
- Antibody: Rabbit anti-P-Stat3
- Dilution 1/200 in Envision FLEX antibody diluent
- 200 µl/sample
- O/N 4 °C
- Antibody: Rabbit anti-P-Stat3
- 3 washes 5 min with 1 ml 1x wash buffer
- Incubation with antibody BrightVision
- Antiboby: BrightVision anti-Rabbit
- 150 µl/sample
- Time: 45 min at room temperature
- Antiboby: BrightVision anti-Rabbit
- 3 washes 5 min with 1 ml 1x wash buffer
- Revealed with ImmPACT DAB.
- 200 µl/sample
- Time: 10 min
- 200 µl/sample
- 3 washes 5 min with 1 ml distilled water.
- Hematoxylin (1 ml) counterstaining, time: 2 min.
- Rinse in distilled water bath.
- Rinse in TAP water bath.
- Dehydration and mounting with DPX.
Representative data
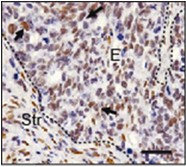
Figure 1. p-STAT3 staining of liver metastases generated after intrasplenic injection of CRC cells. Note strong staining in epithelial cells (arrows). E: epithelial cells, Str: stromal cells. Scale bar = 50 µm
Recipes
- Tris/EDTA (pH 9.0)
12 g Tris
3.7 g EDTA in 10 L MilliQ
Acknowledgments
A.C. and D.V.F.T hold a Juan de la Cierva postdoctoral fellowship, E.E. an FPI PhD fellowship (both from Spanish Ministry of Science and Innovation). This work has been supported by grants from Instituto de Salud Carlos III FEDER (RD09/0076/00036) and the “Xarxa de Bancs de tumors sponsored by Pla Director d’Oncologia de Catalunya (XBTC), to E.B. from the European Research Council (Starting grant - 208488) and Consolider programmes (MICINN), to E.S. and A.C. by the Spanish Ministry of Science and Innovation, to JM by NIH grant CA34610 and to RM by grants PS09/00965 (MICINN) and NanoCoMets (CIBERBBN).
References
- Calon, A., Espinet, E., Palomo-Ponce, S., Tauriello, D. V., Iglesias, M., Cespedes, M. V., Sevillano, M., Nadal, C., Jung, P., Zhang, X. H., Byrom, D., Riera, A., Rossell, D., Mangues, R., Massague, J., Sancho, E. and Batlle, E. (2012). Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 22(5): 571-584.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Calon, A., Espinet, E., Palomo-Ponce, S., Tauriello, D. V. F., Iglesias, M., Céspedes, M. V., Sevillano, M., Nadal, C., Jung, P., Zhang, X. H., Byrom, D., Riera, A., Rossell, D., Mangues, R., Massague, J., Sancho, E. and Batlle, E. (2014). Immunostaining Protocol: P-Stat3 (Xenograft and Mice). Bio-protocol 4(9): e1120. DOI: 10.21769/BioProtoc.1120.
Category
Cancer Biology > General technique > Biochemical assays > Protein analysis
Cell Biology > Tissue analysis > Tissue staining
Biochemistry > Protein > Immunodetection > Immunostaining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








