- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Histochemical Detection of Superoxide and H2O2 Accumulation in Brassica juncea Seedlings
Published: Vol 4, Iss 8, Apr 20, 2014 DOI: 10.21769/BioProtoc.1108 Views: 34659
Reviewed by: Ru ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Quick Method to Quantify Iron in Arabidopsis Seedlings
Chandan Kumar Gautam [...] Wolfgang Schmidt
Mar 5, 2022 3931 Views

Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Chuanfeng Ju [...] Zhenqian Zhang
Mar 5, 2023 2052 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1181 Views
Abstract
Plant cells continually produce reactive oxygen species (ROS) as a by-product of aerobic metabolism. Increased production of ROS occurs under unfavorable conditions imposed by various abiotic and biotic factors. Accumulation of ROS is damaging to various cellular components and macromolecules including plasma membrane, nucleic acids, and proteins and eventually leads to cell death. In this protocol, we describe the histochemical detection of hydrogen peroxide (H2O2) and superoxide (O2-) anion, two of the most important ROS, in Brassica juncea seedlings by using 3,3ʹ-Diaminobenzidine (DAB) and Nitrotetrazolium blue chloride (NBT) as the chromogenic substrate. DAB is oxidized by H2O2 in the presence of peroxidases and produces reddish brown precipitate. NBT reacts with O2- to form a dark blue insoluble formazan compound. The protocol can be used in other plant species and for different plant tissues.
Materials and Reagents
- Brassica juncea seedlings
- Vermiculite
- Absolute ethanol
- 60% Glycerol
- Aluminium foil
- Test tubes
- Measuring cylinder
- Distilled water
- Nitrotetrazolium blue chloride (NBT) (Sigma-Aldrich, catalog number: N6639 )
- 3,3ʹ-Diaminobenzidine (DAB) (Sigma-Aldrich, catalog number: D8001 )
- Sodium phosphate buffer (see Recipes)
- NBT staining solution (see Recipes)
- DAB staining solution (see Recipes)
Equipment
- Paper towel
- Weighing balance
- Aluminium foil
- Magnetic stirrer
- pH meter
- Water bath
Procedure
- Brassica juncea seedlings grown on vermiculite for 15 days at 22 °C, 75 % relative humidity, and a photoperiod of 16 h light and 8 h dark are treated with a stress inducing agent [for e.g. 200 mM NaCl (for salinity stress), 200 mM mannitol (for drought stress), or 20 mM CdCl2 (for heavy metal stress) for 3 days]. The untreated seedlings grown under the same conditions serve as the experimental control.
- Remove the seedlings from the pot and wash them with distilled water to remove any extraneous material associated with the tissues.
- Place the seedlings in test tubes and immerse them in DAB or NBT staining solution for detection of H2O2 or O2-, respectively. Wrap the tubes with aluminium foil and keep overnight at room temperature.
- Drain off the staining solution from the test tubes.
- Remove the chlorophyll for proper visualization of the stain. This is done by immersing the seedlings in absolute ethanol and heating in a boiling water-bath for 10 min (or more if necessary, with intermittent shaking).
- Transfer the seedlings on a paper towel saturated with 60% glycerol.
- Photograph the stained seedlings against a contrast background for proper documentation. H2O2 is visualized as reddish brown stain formed by the reaction of DAB with the endogenous H2O2 (Figure 1a). The O2- content is detected as dark blue stain of formazan compound formed as a result of NBT reacting with the endogenous O2- (Figure 1b).
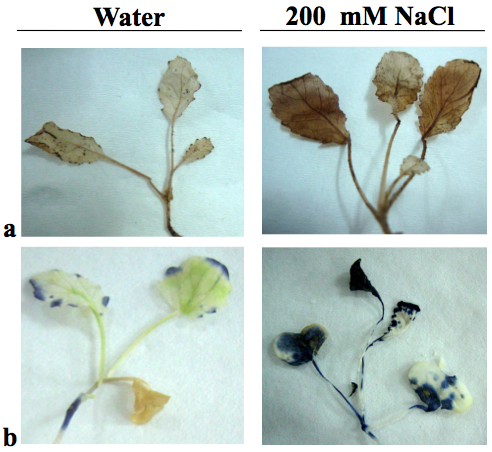
Figure 1. Detection of Hydrogen peroxide (a) and superoxide anion (b) accumulation in Brassica juncea seedlings exposed to 200 mM NaCl stress for 3 days. Seedlings grown in the presence of water served as the untreated control.
Recipes
- Sodium phosphate buffer (pH 7.5)
- Prepare 1 M Sodium phosphate monobasic (NaH2PO4) solution by dissolving 12 g NaH2PO4 in a final volume of 100 ml distilled water.
- Prepare 1 M Sodium phosphate dibasic (Na2HPO4) solution by dissolving 14.2 g Na2HPO4 in a final volume of 100 ml distilled water.
- Mix 16 ml 1 M NaH2PO4 and 84 ml 1 M Na2HPO4 solutions and make the volume to 2 L with distilled water for preparing 50 mM sodium phosphate buffer (pH 7.5).
- Prepare 1 M Sodium phosphate monobasic (NaH2PO4) solution by dissolving 12 g NaH2PO4 in a final volume of 100 ml distilled water.
- DAB staining solution
- Dissolve 50 mg DAB in approximately 45 ml distilled water in an amber colored bottle.
- Adjust the pH to 3.8 using 0.1 N HCl while mixing properly on a magnetic stirrer. On complete dissolution the solution will be clear and light brown in color.
- Make the volume to 50 ml to get 1 mg/ml solution.
- DAB staining solution should be prepared freshly before use.
- Dissolve 50 mg DAB in approximately 45 ml distilled water in an amber colored bottle.
- NBT staining solution
- In an amber colored bottle, dissolve 0.1 g NBT in 50 mM sodium phosphate buffer (pH 7.5) and make the volume to 50 ml to get a 0.2% solution.
- Mix the solution thoroughly using a magnetic stirrer.
- NBT staining solution should be prepared freshly before use.
- In an amber colored bottle, dissolve 0.1 g NBT in 50 mM sodium phosphate buffer (pH 7.5) and make the volume to 50 ml to get a 0.2% solution.
Acknowledgments
The protocol was implemented in a project that was partially funded by the Council of Scientific and Industrial Research (CSIR) Grant No. 38/1126/EMR-II. DK and PS acknowledge the financial support from CSIR and University Grants Commission (UGC). MAY was a UGC-Dr. D.S. Kothari Postdoctoral Fellow during the course of this project. Research in the laboratory of NBS is supported by U.G.C.-C.A.S., U.G.C.-R.N.W., Department of Science and Technology (D.S.T.)-F.I.S.T., and D.S.T.-PURSE. The authors would like to acknowledge Thordal-Christensen et al. (1997) and Jabs et al. (1996) (kindly see the reference section) whose work was adapted in the present protocol.
References
- Jabs, T., Dietrich, R. A. and Dangl, J. L. (1996). Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273(5283): 1853-1856.
- Kumar, D., Yusuf, M. A., Singh, P., Sardar, M. and Sarin, N. B. (2013). Modulation of antioxidant machinery in alpha-tocopherol-enriched transgenic Brassica juncea plants tolerant to abiotic stress conditions. Protoplasma 250(5): 1079-1089.
- Thordal-Christensen, H., Zhang, Z., Wei, Y. and Collinge, D. B. (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187-1194.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kumar, D., Yusuf, M. A., Singh, P., Sardar, M. and Sarin, N. B. (2014). Histochemical Detection of Superoxide and H2O2 Accumulation in Brassica juncea Seedlings. Bio-protocol 4(8): e1108. DOI: 10.21769/BioProtoc.1108.
Category
Plant Science > Plant biochemistry > Other compound
Biochemistry > Other compound > Reactive oxygen species
Cell Biology > Cell staining > Reactive oxygen species
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link














