- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Culture of Neurospheres for the Study of Pathogenesis of Prion Disease
Published: Vol 4, Iss 6, Mar 20, 2014 DOI: 10.21769/BioProtoc.1081 Views: 22472
Reviewed by: Oneil G. BhalalaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Derivation and Culture of Enriched Phrenic-Like Motor Neurons From Human iPSCs
Louise Thiry [...] Stefano Stifani
Jul 5, 2025 2289 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 230 Views
Abstract
Neurosphere contains neural stem cells that are capable of self-renewal and multilineage differentiation including neurons, astrocytes, and oligodendrocytes (Gage, 2000). Cell culture model using differentiated neurosphere cultures are suggested to be a valuable tool for studying the pathogenesis of prion disease at the cellular level (Iwamaru et al., 2013). This protocol describes the procedure for a culture of whole brain-derived neurospheres from newborn mouse brains. Neurosphere formation steadily occurs within a week from the cultures of neonatal whole brains and these cells have stem cell properties.
Keywords: Neural stem cellMaterials and Reagents
- Newborn mice (age at one day after birth)
Present culture method can be applied to any mouse strains, regardless of sex. We used prion protein overexpressing transgenic mouse and prion protein-deficient mouse for our present study (Iwamaru et al., 2013).
- Dulbecco's phosphate buffered saline without Ca and Mg (D-PBS) (Nacalai Tesque, catalog number: 14249-95 )
- Di‐sodium dihydrogen ethylenediamine tetraacetate dihydrate (EDTA) (Nacalai Tesque, catalog number: 151-11 )
- Hank's balanced salt solution (HBSS) (Sigma-Aldrich, catalog number: H8264 )
- Dulbecco's modified Eagle's medium/nutrient mixture F-12 Ham (DMEM/F12 Ham) (Sigma-Aldrich, catalog number: D8437 )
- N-2 supplement (Life Technologies, catalog number: 17502-048 )
- Penicillin-Streptomycin (Sigma-Aldrich, catalog number: P0781 )
- Fetal bovine serum (FBS) (Hyclone, catalog number: SH30070.03 )
- Accutase (Innovative Cell Technologies, catalog number: AT104 )
- Trypsin (Sigma-Aldrich, catalog number: T8003 ) (see Recipes)
- Deoxyribonuclease I (DNase I) (Worthington Biochemical Corporation, catalog number: LS002139 ) (see Recipes)
- Epidermal growth factor human (EGF) (Sigma-Aldrich, catalog number: E9644 ) (see Recipes)
- Fibroblast growth factor-basic human (bFGF) (Sigma-Aldrich, catalog number: F0291 ) (see Recipes)
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9576 ) (see Recipes)
- Neurosphere basal medium (see Recipes)
Equipment
- Falcon 15 ml conical centrifuge tube (Corning, catalog number: 352096 )
- Falcon 100 mm cell culture dish (Corning, catalog number: 353003 )
- Nunc 60 mm dish with HydroCell surface (Thermo Fisher Scientific, catalog number: 174912 )
- Disposable micro homogenizer (TaKaRa Bio, TaKaRa BioMasher Standard, catalog number: 9791A )
- Aerosol resistant tip (ART 1000 REACH tips) (Thermo Fisher Scientific, model: 2079 )
- Membrane filter (EMD Millipore, Millex-GV 0.22 μm, 33 mm)
- Dissecting microscope (Olympus, model: SZH10 )
- Centrifuge (KUBOTA, model: 5220 )
- CO2 incubator (set at 37 °C and 5% CO2-95% air)
- Reciprocal shaker (TAITEC, model: NR-1 )
- Laminar flow hood (W800 x H1050 x D500 mm) (Panasonic Corporation, SANYO, model: MCV-710ATS )
- Scissors (NAPOX B-5H, 154 mm; NAPOX B-1112H, 110 mm), fine forceps (NAPOX A-5, 110 mm; NAPOX MA-45, 110 mm), spatula (Laboran 9-891-03, 180 mm)
- Ice bucket
Procedure
- Dissect the brain from newborn mice under Laminar flow hood (Figure 1). Spray 70% ethanol upon newborn mice and cut the head (Figure 2A). Make small incisions (Figure 2B, arrows) and remove the skin. Then, carefully remove the skull with forceps and expose the brain (Figure 2C).
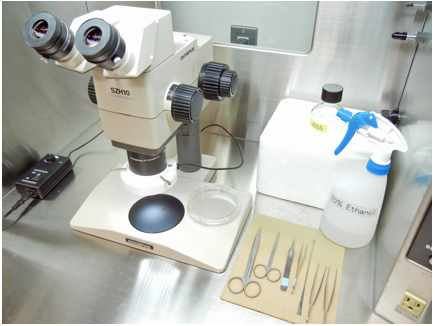
Figure 1. Dissecting equipments arranged in a Laminar flow hood
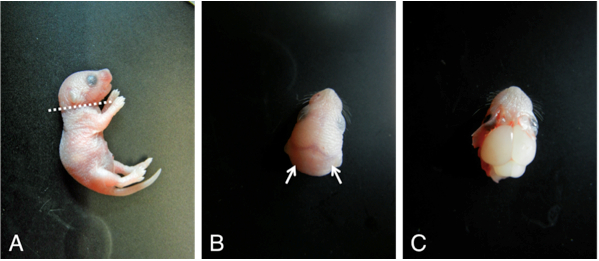
Figure 2. Dissection procedures to obtain neonatal mouse brain
- Scoop the brain by a small spatula and place them in ice cold HBSS (10 ml in 100 mm dish, Figure 3A). By using fine forceps, remove meninges under dissecting microscope (Figure 3B-C).
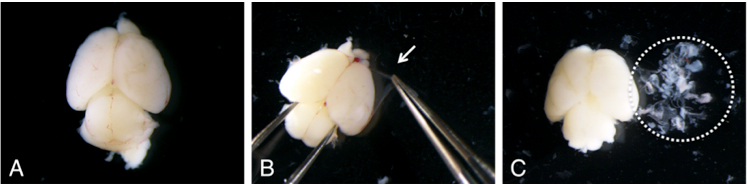
Figure 3. Removal of meninges (B: arrow) from neonatal mouse brain under dissecting microscope. After removal of meninges, the brain becomes whitish color (C).
- Place cortices in sterilized disposable micro homogenizer and centrifuge at 5000 x g for 30 sec at 4 °C.
- Pipet the minced tissue with 2 ml of D-PBS containing 1 mM EDTA, 100 μg/ml trypsin, and 400 μg/ml DNase I until evenly homogenized and place them in a 15 ml Conical Centrifuge Tube.
- Incubate for 15 min at 37 °C in an incubator with constant agitation using a reciprocal shaker at 50 rpm. Add 1 ml of FBS, then gently pipet up and down to help the dissociation by using aerosol resistant tip to avoid contamination. Stand tubes for 2 min at 25 °C and collect supernatant by pipetting. Avoid to suck any visible tissue fragments.
- Centrifuge cell suspensions at 200 × g for 5 min at 25 °C and resuspend the pellet in Neurosphere basal medium (10 ml) by pipetting.
- Centrifuge the cell suspension at 200 x g for 5 min at 25 °C. Discard the supernatant, resuspend in 5 ml of Neurosphere basal medium at 25 °C and transfer it into Nunc 60 mm dishes with HydroCell Surface. Culture the cells at 37 °C in humidified CO2 incubator (5% CO2-95% air).
- Refill 0.5 ml of Neurosphere basal medium everyday.
- After 5-7 days, neurospheres with variable sizes (50-100 μm in diameter) are formed in the culture (Figure 4). Centrifuge neurosphere suspensions at 200 × g for 5 min at 25 °C, discard the supernatant, and resuspend in 2 ml of Accutase at 37 °C for 10 min.
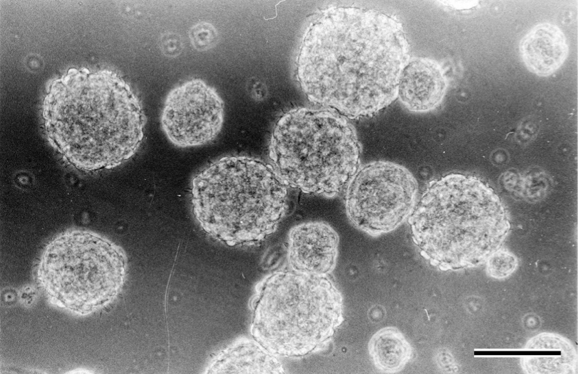
Figure 4. Neurosphere formation in Neurosphere basal medium after 5 days of culture. Scale bar = 100 μm
- Gently pipet up and down (about ten times) using aerosol resistant tip to dissociate into single cells. Check the cells under microscope for successful dissociation. Add 12 ml of HBSS and centrifuge at 200 x g for 5 min at 25 °C. Resuspend the pellet in 10 ml of Neurosphere basal medium at 25 °C and subculture into new Nunc 60mm dishes with HydroCell Surface at 1:4 split ratio (i.e. 25% of the harvested cells are seeded into the new dish with same size). Refill 0.5 ml of Neurosphere basal medium everyday. The neurospheres are subcultured at 4-6 day intervals.
Recipes
- Deoxyribonuclease I (DNase I)
Dissolved at 4 mg /ml in D-PBS
Sterile filtered (0.22 μm)
Aliquoted (4 ml) and stored at -20 °C
Use within 6 months
- Epidermal growth factor human (EGF)
Dissolved at 50 μg /ml in sterile DMEM/F12 Ham containing 1% BSA
Aliquoted (100 μl) and stored at -20 °C
Use within 6 months
- Fibroblast growth factor-basic human (bFGF)
Dissolved at 50 μg /ml in sterile DMEM/F12 Ham containing 1% BSA
Aliquoted (50 μl) and stored at -20 °C
Use within 6 months
- Bovine serum albumin (BSA)
Dissolved at 300 mg /ml in D-PBS
Sterile filtered (0.22 μm)
Stored at 4 °C
Use within 6 months
- Neurosphere basal medium
Reagents
Volume
Final conc.
DMEM/F12 Ham
487.33 ml
EGF
500 μl
50 ng/ml
bFGF
500 μl
50 ng/ml
BSA
1.67 ml ml
0.1%
N2 supplement
5 ml
1%
Penicillin-streptomycin
5 ml
100 IU/ml and 100 μg/ml, respectively
- Trypsin
Dissolved at 1 mg /ml in D-PBS containing 10 mM EDTA
Sterile filtered (0.22 μm)
Aliquoted (4 ml) and stored at -20 °C
Use within 6 months
For the dissociation of minced brain tissue, mix the reagents as follows:Reagents
Volume
Final conc.
Trypsin
1 ml
100 μg/ml
DNase I
1 ml
400 μg/ml
D-PBS
8 ml
Acknowledgments
This protocol was adapted from our previously published paper: Iwamaru et al. (2013). This work was supported by a Grant-in-Aid from the BSE and Other Prion Disease Control Project of the Ministry of Agriculture, Forestry, and Fisheries of Japan and by a Grant-in-Aid for Young Scientists (category B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Gage, F. H. (2000). Mammalian neural stem cells. Science 287(5457): 1433-1438.
- Iwamaru, Y., Takenouchi, T., Imamura, M., Shimizu, Y., Miyazawa, K., Mohri, S., Yokoyama, T. and Kitani, H. (2013). Prion replication elicits cytopathic changes in differentiated neurosphere cultures. J Virol 87(15): 8745-8755.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Iwamaru, Y., Takenouchi, T. and Kitani, H. (2014). Isolation and Culture of Neurospheres for the Study of Pathogenesis of Prion Disease. Bio-protocol 4(6): e1081. DOI: 10.21769/BioProtoc.1081.
Category
Neuroscience > Development > Neuron
Stem Cell > Adult stem cell > Neural stem cell
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









