- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Human Astrovirus Propagation, Purification and Quantification
Published: Vol 4, Iss 6, Mar 20, 2014 DOI: 10.21769/BioProtoc.1078 Views: 12114
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Detachment Procedure of Bacteria from Atmospheric Particles for Flow-cytometry Counting
Carolina M. Araya [...] Isabel Reche
Jun 20, 2019 5593 Views

General Maintenance and Reactivation of iSLK Cell Lines
Ariana C. Calderón-Zavala [...] Ekaterina E. Heldwein
Jun 5, 2025 1896 Views
Abstract
Astrovirus are small, nonenveloped, single-stranded RNA viruses that cause diarrhea in a wide variety of mammals and birds. Here, we describe astrovirus propagation, purification and titration. The Caco-2 human intestinal adenocarcinoma cell line is most widely used for studying astrovirus, although other cell lines, such as 293, T84 and LLC-MK2 can be used for propagation. However, Caco2 cells are desirable for their ability to form a differentiated intestinal epithelium, mimicking the human intestine and providing a realistic model for astrovirus growth and propagation.
Keywords: AstrovirusMaterials and Reagents
- Caco2 cell line (ATCC, catalog number: HTB-37 )
- MEM (Mediatech, Cellgro®, catalog number: 10-010-CV , or equivalent)
- Glutamax (Life Technologies, Gibco®, catalog number: 35050-061 , or equivalent),
- Sodium pyruvate (Life Technologies, Gibco®, catalog number: 11360-070 , or equivalent)
- 10% heat-inactivated FBS
- Porcine type 1x trypsin (Sigma-Aldrich, catalog number: T-0303 , or equivalent)
- MgCl2
- Sucrose
- Phosphate buffered saline (PBS)
- Bovine serum albumin Fraction V (BSA) (Life Technologies, Gibco®, catalog number: 15260-037 )
- Formaldehyde (Polysciences, catalog number: 18814 )
- TritonX-100 (Sigma-Aldrich, catalog number: 9002-93-1 )
- Normal goat serum (Sigma-Aldrich, catalog number: 191356 )
- 4',6-diamidino-2-phenylindole (DAPI) (Life Technologies, Molecular Probes®, catalog number: D1306 )
- Astrovirus 8E7 mouse monoclonal antibody (hybridoma cell line) (ATCC, catalog number: HB-11945 , or equivalent)
- Fluorescent-conjugated anti-mouse IgG antibody [Life Technologies, Alexa Fluor® 488 Goat Anti-Mouse (H+L) Antibody, catalog number: A11001 , or equivalent]
- Astrovirus (not commercially available)
- Bleach (clorox or stored brand equivalent)
- Virkon S (DuPont)
- Caco2 cell culture medium (see Recipes)
- Serum-free (SF) Caco2 medium (see Recipes)
- TN buffer (see Recipes)
Equipment
- Biosafety cabinet
- Gloves
- Labcoat
- Beckman ultracentrifuge tubes (ultra-clear 9/16 x 3 ½ in) (Beckman Coulter, catalog number: 344059 )
- Beckman ultracentrifuge (that can reach 34,000 rpm and 4 ºC)
- SW41 rotor
- Pierce Extra-Strength Slide-A-Lyzer 10K molecular weight cassette (Pierce, catalog number: 66383 or 66380 )
- Laminar flow hood
- Syringe
- Liquid nitrogen
- Water bath
Procedure
- Propagating human Astrovirus
- Seed T-75 flask(s) with 2.5-5 x 106 Caco2 cells in cell culture medium. Grow at 37 ºC in 5% CO2 for 3-5 days until cells reach 100% confluence.
- Rinse confluent Caco2 cells 1x with 3-4 ml PBS.
- Incubate cells for 1 h at 37 ºC, 5% CO2 in SF Caco2 Medium containing 5 µg/ml porcine trypsin.
- All subsequent steps should be performed in a certified biosafety cabinet by personnel wearing disposable gloves and a labcoat working under BL2 conditions.
- Remove media and infect cells with astrovirus in 5 ml SF Caco2 Medium with 5 µg/ml porcine trypsin for 90 min at 37 ºC, 5% CO2 (see Notes 1 and 2).
- Remove the infection media and replace with 6 to 7 ml SF Caco2 Medium with 10 µg/ml porcine trypsin (see Note 3). Use extreme caution as the cells will begin to detach due to the trypsin.
- Incubate at 37 ºC, 5% CO2 for 3 to 4 days, then collect supernatant and cells.
- To increase yield, sonicate 4 x 15 sec. Alternatively, pellet cells by centrifuging for 5 min at 4,000 rpm. Remove all but 1 ml of supernatant and freeze-thaw the cell pellet 3 times by freezing in liquid nitrogen and thawing at 37 ºC. Centrifuge again to pellet cell debris and combine supernatants.
- Aliquot the virus and stored at -80 ºC.
- Seed T-75 flask(s) with 2.5-5 x 106 Caco2 cells in cell culture medium. Grow at 37 ºC in 5% CO2 for 3-5 days until cells reach 100% confluence.
- Purification of Astrovirus
- Pre-clear astrovirus solution by centrifuging at 4,000 rpm to pellet cellular debris.
- For each tube
- Pipet 4 ml of 15% sucrose (w/v) in TN buffer into a 12 ml Beckman ultra-clear 9/16 x 3 ½ in ultracentrifuge tube.
- Underlay with 2 ml of 50% sucrose (w/v) in TN buffer.
- Pipet approximately 6 ml of pre-cleared astrovirus supernatant (or PBS/TN buffer if a balance is needed) on top of the 15% sucrose layer (Figure 1A).
- Pipet 4 ml of 15% sucrose (w/v) in TN buffer into a 12 ml Beckman ultra-clear 9/16 x 3 ½ in ultracentrifuge tube.
- Spin in SW41 ultracentrifuge rotor at 34,000 rpm for 3 h at 4 °C.
- Remove virus by puncturing the side of the tube with a syringe just under the virus band (white cloudy band at the 15%/50% interface; Figure 1B; see Note 4).
- Insert virus into Slide-A-Lyzer 10K dialysis cassette. Remove excess air in the cassette.
- Dialyze virus in PBS + 10 mM MgCl2 for at least 4 h with 2 buffer changes (2 L total) or overnight in 2 L buffer at 4 ºC.
- Remove virus using a syringe. Aliquot and freeze at -80 ºC.
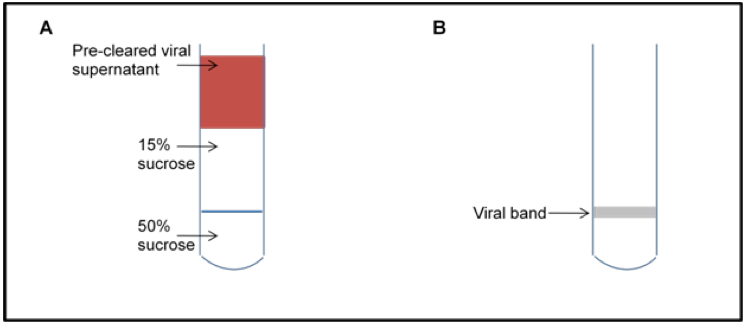
Figure 1. Schematic of sucrose gradient. Schematic of gradient loading (A) and the viral band after centrifugation (B).
- Pre-clear astrovirus solution by centrifuging at 4,000 rpm to pellet cellular debris.
- Astrovirus Fluorescent Focus Assay
- Seed a 96-well plate with 2 x 104 Caco2 cells/well. Grow 3-4 days until cells reach 100% confluence.
- Gently rinse cells 2x with sterile PBS. Trypsinize and count 2-3 wells to calculate the number of cells/well.
- Add 100 µl of SF Caco2 media (supplemented with 0.3% BSA) and incubate for 1 h at 37 °C, 5% CO2.
- Prepare ten-fold viral dilutions in SF Caco2 media containing 0.3% BSA.
- Remove media from cells and add 100 µl of serial dilutions to cells. Each sample should be assayed in triplicate.
- Incubate 1 h at 37 °C, 5% CO2. Remove media and replace with SF Caco2 media containing 0.3% BSA.
- Incubate plate at least 10 h at 37 °C, 5% CO2, but not more than 24 h.
- Remove media. Rinse cells gently 3x with PBS.
- Fix in 100 µl of 4% formaldehyde/PBS at room temperature for 20 min.
- Rinse 3x with ~100-200 µl of PBS. All rinses should be performed with this approximate volume.
- Permeabilize for 10 min at room temperature with 100 µl of PBS containing 0.5% TritonX-100.
- Rinse 3x with PBS.
- Block 1 h in 100 µl of 5% normal goat serum in PBS at room temperature. Plate can be gently rocked if desired.
- Rinse 3x with PBS.
- Incubate with primary mouse anti-HAstV-1 capsid protein (8E7) 1:100 in 50 µl 1% normal goat serum in PBS for 1-2 h at room temperature or overnight at 4 °C. Plate can be gently rocked if desired.
- Rinse 3x with PBS.
- Incubate with secondary goat anti-mouse AlexaFluor 488 1:100 in 50 µl 1% normal goat serum in PBS+ 1 µg/ml DAPI. Keep plate away from light from this step forward. Plate can be gently rocked if desired.
- Rinse 3x with PBS. Add ~200 µl PBS to each well and visualize on fluorescent microscope (Figure 2).
- Calculate fluorescent focus units per ml (FFU/ml): %FITC+ cells x average number of cells/well x dilution factor = FFU/Ml
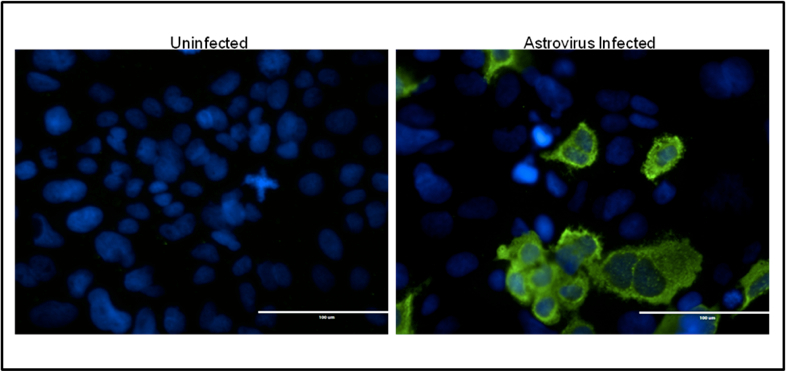
Figure 2. Example of Astrovirus staining. IFA staining of uninfected (left) and astrovirus infected (right) Caco2 cells.Astrovirus capsid is shown in green, and cell nuclei in blue.
- Seed a 96-well plate with 2 x 104 Caco2 cells/well. Grow 3-4 days until cells reach 100% confluence.
Notes
- For propagating astrovirus stocks that were made following this protocol, infect with an MOI: 1 (if possible). If using fecal filtrate or stocks that have not been grown in the presence of porcine trypsin, pre-incubate filtrate/stock with 10 µg/ml porcine trypsin for 1 h before adsorption onto Caco2 cells.
- During the 90 min infection, rock cells every 15 min.
- Inactivate virus in inoculum by incubating in 10% vol/vol bleach for at least 30 min before disposal; autoclaving inoculum; or incubating in 1% vol/vol Virkon S for 30 min.
- Visualization of the viral band can be increased by either placing a black background behind the ultracentrifuge tube, or by turning the lights off in the hood/room and shining light at the band.
Recipes
- Caco2 cell culture medium
MEM supplemented with:
1% glutamax
1% sodium pyruvate
10% heat-inactivated FBS - Serum-free (SF) Caco2 medium
MEM supplemented with:
1% glutamax
1% sodium pyruvate - TN buffer
50 mM Tris (pH 7.5)
100 mM NaCl
Sterilized
Acknowledgments
This protocol was adapted from the previous publications DuBois et al. (2013); Moser and Schultz-Cherry (2008); and Willcocks et al. (1990). Funding for this research was provided by the Children’s Infection Defense Center, the Hartwell Foundation, and the American Lebanese Syrian Associated Charities and St Jude Children’s Research Hospital.
References
- DuBois, R. M., Freiden, P., Marvin, S., Reddivari, M., Heath, R. J., White, S. W. and Schultz-Cherry, S. (2013). Crystal structure of the avian astrovirus capsid spike. J Virol 87(14): 7853-7863.
- Moser, L. A. and Schultz-Cherry, S. (2008). Suppression of astrovirus replication by an ERK1/2 inhibitor. J Virol 82(15): 7475-7482.
- Willcocks, M., Carter, M., Laidler, F. and Madeley, C. (1990). Growth and characterisation of human faecal astrovirus in a continuous cell line. Arch Virol 113(1-2): 73-81.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Marvin, S., Meliopoulos, V. and Schultz-Cherry, S. (2014). Human Astrovirus Propagation, Purification and Quantification. Bio-protocol 4(6): e1078. DOI: 10.21769/BioProtoc.1078.
Category
Microbiology > Microbial cell biology > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










