- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Adenosine A2A Receptor Ligand Binding Experiments by Using Real-time Single-cell FRET
Published: Vol 4, Iss 6, Mar 20, 2014 DOI: 10.21769/BioProtoc.1070 Views: 11014
Reviewed by: Cheng Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Enrichment of Membrane Proteins for Downstream Analysis Using Styrene Maleic Acid Lipid Particles (SMALPs) Extraction
Benedict Dirnberger [...] Kathryn S. Lilley
Aug 5, 2023 2978 Views

Establishment of Human PD-1/PD-L1 Blockade Assay Based on Surface Plasmon Resonance (SPR) Biosensor
Tess Puopolo [...] Chang Liu
Aug 5, 2023 2842 Views

A Computational Workflow for Membrane Protein–Ligand Interaction Studies: Focus on α5-Containing GABA (A) Receptors
Syarifah Maisarah Sayed Mohamad [...] Ahmad Tarmizi Che Has
Nov 20, 2025 2123 Views
Abstract
We designed a fluorescence resonance energy transfer (FRET)-based approach to study the ligand binding constants of the adenosine A2A receptor (A2AR). Our assay is based in the interaction of a fluorescent A2AR agonist ligand (MRS5424) with an A2AR tagged with the cyan fluorescent protein (CFP) at the N-terminus (i.e. A2ARCFP) and expressed in living cells. Thus, upon fast superfusion of the A2ARCFP expressing cells with MRS5424, the ligand-receptor interaction is determined by single-cell FRET in a real-time mode. Accordingly, our approach allowed immediate ‘real-time’ readout of the ligand-receptor interaction, thus allowing kinetic binding experiments, a feature impossible to achieve using conventional radioisotope-labelled ligands. In addition, since our assay permitted the visual confirmation of receptor localization it also allowed localized saturation binding experiments.
Keywords: FRETMaterials and Reagents
- Cell line (i.e. HEK-293 cells)
- Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich)
- Sodium pyruvate
- L-glutamine
- Antibiotics: streptomycin and penicillin
- Fetal bovine serum
- TransFectinTM Lipid Reagent (Bio-Rad Laboratories)
- Hank’s balanced salt solution (HBSS) (see Recipes)
- Cell culture medium (see Recipes)
Equipment
- 18 mm diameter glass coverslips
- Attofluor holder
- Inverted Axio Observer microscope (ZEISS) equipped with a 63x oil immersion objective
- Polychrome V (TILL Photonics)
- Avalanche photodiodes (TILL Photonics)
- Focal drug application system (ALA Scientific Instruments, OCTAFLOWTM)
- Digidata 1440A analog/digital converter (Molecular Devices)
Software
- pCLAMP (Molecular Devices)
- GraphPad Prism (GraphPad Software)
Procedure
- Two days before the experiment, the cells (i.e. HEK-293 cells) were seeded onto 18 mm diameter glass coverslips and transiently transfected with an A2AR construct tagged with the CFP at its N-terminal tail (A2ARCFP) (Figure 1).
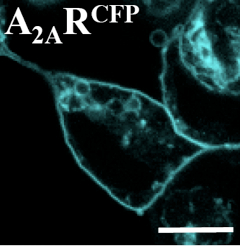
Figure 1. Cell surface localisation of the A2ARCFP construct. HEK-293 cells were transiently transfected with A2ARCFP, fixed and analyzed by confocal microscopy. The A2ARCFP was mainly targeted to the cell surface and scarcely accumulated at the intracellular level (Fernández-Dueñas et al., 2013). Scale bar: 10 µm
- The day of the experiment the transiently transfected cells were mounted in an Attofluor holder and placed on an inverted Axio Observer microscope equipped with a 63x oil immersion objective and a dual-emission photometry system.
- Then, cells were continuously superfused with a FRET-compatible A2AR fluorescent ligand (i.e. MRS5424) (Fernández-Dueñas et al., 2012) dissolved in HBSS and applied with the aid of a focal drug application system.
- A Polychrome V was used as the light source in our dual-emission photometry system. Upon excitation with the corresponding donor excitation wavelength (i.e. A2ARCFP) the fluorescent signals of the donor and acceptor fluorophores were detected by avalanche photodiodes and digitized using a Digidata 1440A analog/digital converter.
- pCLAMP and GraphPad Prism softwares were used for data collection and analysis.
- Accordingly, a FRET signal was measured upon donor (i.e. A2ARCFP) excitation at 430 ± 10 nm [beam splitter dichroic long-pass (DCLP) 460 nm] and an illumination time set to 10 ms at 10 Hz. Then, the emission light intensities were determined at 535 ± 15 nm (F535; MRS5424 emission) and 480 ± 20 nm (F480; A2ARCFP emission) with a beam splitter DCLP of 505 nm. No corrections for spillover between channels or direct MRS5424 excitation were made.
- The increase in FRET ratio (F535/F480) was fitted to the equation: r(t) = A x (1 - e-t/τ), where τ is the time constant (in seconds) and A is the magnitude of the FRET signal (Figure 2). When necessary for calculating τ, agonist-independent changes in FRET due to photobleaching were subtracted (Fernández-Dueñas et al., 2012, Fernández-Dueñas et al., 2013).
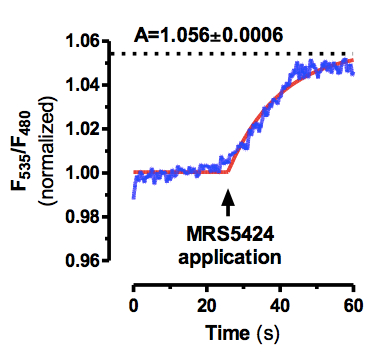
Figure 2. Example of FRET ratio fitting. Time-resolved changes in A2ARCFP and MRS5424 fluorescence emission signals in single cells transfected with A2ARCFP (see Figure 1). The ratio (blue trace) of the emission intensities of the MRS5424 (F535) and CFP (F480) in response to MRS5424 application was recorded from single HEK293 cells expressing the A2ARCFP (see Figure 1). Shown are the changes induced by rapid superfusion with 2 μM MRS5424. The increase of the ratio F535/F480 was fitted by a simple monoexponential curve (r(t) = A x (1 - e-t/τ)) using the GraphPad Prism software which gave a time constant (τ) in this experiment of 14 ± 1 sec. This assay is well suited for competitive ligand binding experiments using non-fluorescent compounds (Fernández-Dueñas et al., 2012, Fernández-Dueñas et al., 2013).
Recipes
- HBSS
137 mM NaCl
5.4 mM KCl
0.3 mM Na2HPO4
0.4 mM KH2PO4
4.2 mM NaHCO3
1.3 mM CaCl2
0.5 mM MgCl2
0.6 mM MgSO4
5.6 mM glucose
pH 7.4
- Cell culture medium
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with:
1 mM sodium pyruvate
2 mM L-glutamine
100 U/ml streptomycin
100 mg/ml penicillin
5% (v/v) fetal bovine serum
Acknowledgments
This work was supported by grants SAF2011-24779, Consolider-Ingenio CSD2008-00005 and PCIN-2013-019-C03-03 from Ministerio de Economía y Competitividad and ICREA Academia-2010 from the Catalan Institution for Research and Advanced Studies (to FC), by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Intramural Research Program (to KAJ). FC belong to the “Neuropharmacology and Pain” accredited research group (Generalitat de Catalunya, 2014 SGR 1251). We thank E. Castaño and B. Torrejón from the Scientific and Technical Services (SCT) group at the Bellvitge Campus of the University of Barcelona for their technical assistance.
References
- Fernandez-Duenas, V., Gomez-Soler, M., Jacobson, K. A., Kumar, S. T., Fuxe, K., Borroto-Escuela, D. O. and Ciruela, F. (2012). Molecular determinants of A2AR-D2R allosterism: role of the intracellular loop 3 of the D2R. J Neurochem 123(3): 373-384.
- Fernández-Dueñas, V., Gómez-Soler, M., Morato, X., Núñez, F., Das, A., Kumar, T. S., Jaumà, S., Jacobson, K. A. and Ciruela, F. (2013). Dopamine D2 receptor-mediated modulation of adenosine A2A receptor agonist binding within the A2AR/D2R oligomer framework. Neurochem Inter 63(1): 42-46.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Fernández-Dueñas, V., Jacobson, K. A. and Ciruela, F. (2014). Adenosine A2A Receptor Ligand Binding Experiments by Using Real-time Single-cell FRET. Bio-protocol 4(6): e1070. DOI: 10.21769/BioProtoc.1070.
Category
Neuroscience > Cellular mechanisms > Receptor-ligand binding
Biochemistry > Protein > Interaction > Protein-ligand interaction
Cell Biology > Single cell analysis > Cell carrier
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










