- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Limiting Dilution Assays to Determine Frequencies of Lymphohematopoietic Progenitors
Published: Vol 4, Iss 4, Feb 20, 2014 DOI: 10.21769/BioProtoc.1045 Views: 10298
Reviewed by: Omar AkilAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Quantification of Mouse γδT-cells in vitro and in vivo
Isha Rana [...] Colin Jamora
Sep 5, 2021 5007 Views

Expansion and Polarization of Human γδT17 Cells in vitro from Peripheral Blood Mononuclear Cells
Xu Chen [...] Jun Yan
Jan 5, 2024 1951 Views

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2620 Views
Abstract
This protocol is useful to determine the frequencies of lymphohematopoietic progenitors in tested samples. To effectively support the growth and differentiation of primitive lymphohematopoietic progenitors, complex signals from stromal cells are important. Several stromal cell lines are known to support both lymphoid and myeloid cells simultaneously in mouse. In this protocol, we introduce two stromal co-culture systems for murine lymphohematopoietic progenitors and their application for limiting dilution assays.
Materials and Reagents
- Stromal cells (MS5 cells or OP9 cells)
- Growth factors
a. rm SCF (10 ng/ml) (e.g. R&D systems, catalog number: 455-MC-010 )
b. rm Flt3-ligand (20 ng/ml) (e.g. R&D systems, catalog number: 427-FL-025 )
c. rm IL-7 (1 ng/ml) (e.g. R&D systems, catalog number: 407-ML-005 )
- Antibodies
a. Anti-mouse Mac1 (BD Biosciences, catalog number: 557396 )
b. Gr1 (BD Biosciences, catalog number: 553128 )
c. CD19 (BD Biosciences, catalog number: 557399 )
d. CD45R/B200 (BD Biosciences, catalog number: 553092 )
e. CD45 (BD Biosciences, catalog number: 550994 )
- Trypsin/EDTA solution (Nacalai Tesque, catalog number: 35554-64 )
- EDTA solution (Nacalai Tesque, catalog number: 14367-74 )
- Alpha Modification of Eagle’s Medium (Corning, cellgro®, catalog number: 50-012 )
- Minimum Essential Medium Alpha Medium (Gibco®, catalog number: 41061-029 )
- FCS (MP Biomedicals, catalog number: 2916754 )
- L-glutamine (Corning, cellgro®, catalog number: 61-030 )
- Penicillin-Streptomycin (Nacalai Tesque, catalog number: 26253-84 )
- MS-5 growth media (see Recipes)
- OP-9 growth media (see Recipes)
Equipment
- Cell sorting machines (BD Biosciences, FACSAriaTM) with the automated cell deposition system
- 96-well flat bottom plates
- 10 cm plastic dishes
- CO2 incubators
- Flow cytometry system
Procedure
- Grow stromal cells to a sub-confluent state in 10 cm-dishes. Use MS-5 growth media to grow MS-5 stromal cells. Use OP-9 growth media to grow OP-9 stromal cells.
- Wash the stromal cells twice with PBS and trypsinize with trypsin/EDTA solution at 37 °C for 5 min. Dissociate the cells by pipetting. After centrifugation at 300 x g for 5 min, re-suspend the cells in the growth medium. Seed half of the cells from a 10 cm dish into one 96-well plate on a day before the start of co-culture. The density and volume of stromal cells are 40~50 cells/ml and 50 ml/well when they are seeded into 96-well plates.
- Prepare testing lymphohematopoietic progenitors. (The testing progenitors should differ according to the aim of each experiment.) Put the progenitors with the different densities (for example 1, 2, 4, 8, 16, 32, 64 cells per well) onto the prepared stromal layers by using a cell-sorting machine. Culture the progenitors on stromal cells in alpha Modification of Eagle’s Medium supplemented with 10% FCS, rmSCF (10 ng/ml), rmFlt3-ligand (20 ng/ml), and rmIL7 (1 ng/ml).
- Feed the cultures twice a week by removing half of the medium and replacing it with fresh medium. Cytokines should be freshly added with each feeding.
- At 10 days of culture, inspect the wells for the presence of hematopoietic clones. Positive wells are harvested and analyzed by flow cytometry for the presence of CD45+ hematopoietic cells, CD45R/B220+ CD19+ Mac1- (and/or Gr1-) B-lineage cells, and Mac1+ (and/or Gr1+) myeloid-lineage cells (Figure 1).
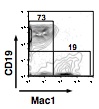
Figure 1. Shown above is a representative profile of flow cytometry showing the expression of Mac1 and CD19 on recovered CD45+ cells (from Yokota et al., 2009)
- Determine the frequencies of progenitors by calculating with linear regression analysis on the basis of Poisson distribution. The frequencies are given as the reciprocal of the concentration of test cells that result in 37% negative cultures. See Figure 2 for an example.
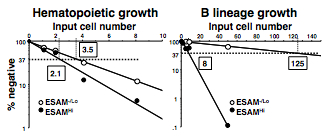
Figure 2. Semilogarithmic plots for hematopoietic cells and B lineage cells are made of % of negative cultures as a function of the dose of input ESAM-/Lo or ESAM+ progenitors in each well. One in 2.1 ESAMHi cells and 1 in 3.5 ESAM-/Lo cells gave rise to CD45+ blood cells, indicating that both populations are potent sources of hematopoietic progenitors (left). However, while 1 in 8 ESAMHi cells gave rise to CD19+ B lineage cells, only 1 in 125 ESAM-/Lo cells produced B lineage cells under these conditions (right) (from Yokota et al., 2009).
Recipes
- MS-5 growth media
Alpha Modification of Eagle’s Medium
10% FCS
2 mM L-glutamine
Penicillin-Streptomycin
- OP-9 growth media
Minimum Essential Medium Alpha Medium
20% FCS
Penicillin-Streptomycin
Acknowledgments
This protocol was adapted from our previously published papers Satoh et al. (2013) and Yokota et al. (2009). The representative data shown in the protocol was adapted from Yokota et al. (2009).
References
- Satoh, Y., Yokota, T., Sudo, T., Kondo, M., Lai, A., Kincade, P. W., Kouro, T., Iida, R., Kokame, K., Miyata, T., Habuchi, Y., Matsui, K., Tanaka, H., Matsumura, I., Oritani, K., Kohwi-Shigematsu, T. and Kanakura, Y. (2013). The Satb1 protein directs hematopoietic stem cell differentiation toward lymphoid lineages. Immunity 38(6): 1105-1115.
- Yokota, T., Oritani, K., Butz, S., Kokame, K., Kincade, P. W., Miyata, T., Vestweber, D. and Kanakura, Y. (2009). The endothelial antigen ESAM marks primitive hematopoietic progenitors throughout life in mice. Blood 113(13): 2914-2923.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Yokota, T. and Satoh, Y. (2014). Limiting Dilution Assays to Determine Frequencies of Lymphohematopoietic Progenitors. Bio-protocol 4(4): e1045. DOI: 10.21769/BioProtoc.1045.
Category
Stem Cell > Adult stem cell > Stromal cell
Immunology > Immune cell isolation > Lymphocyte
Immunology > Immune cell isolation > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











