- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Biomineralization Assay
Published: Vol 4, Iss 3, Feb 5, 2014 DOI: 10.21769/BioProtoc.1036 Views: 19464
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Determination of the Intracellular Calcium Concentration in Peritoneal Macrophages Using Microfluorimetry
Silvia González-Ramos [...] Lisardo Boscá
Dec 5, 2013 18117 Views

Cation (Ca2+ and Mn2+) Partitioning Assays with Intact Arabidopsis Chloroplasts
Anna Harms [...] Anja Schneider
Jan 5, 2017 9030 Views

Quantitative Determination of Ca2+-binding to Ca2+-sensor Proteins by Isothermal Titration Calorimetry
Seher Abbas and Karl-Wilhelm Koch
Apr 5, 2020 6317 Views
Abstract
Biomineralization in vertebrates has both physiological and pathological aspects. Physiological mineralization is essential for proper development and function of hard tissues, such as bone, teeth, and growth plate cartilage, but it does not occur in soft tissues. Pathological ectopic mineralization, in contrast, occurs in soft tissues, including blood vessels, kidney, articular cartilage, and cardiovascular tissue. Here, we describe the simple method for detecting and measuring the presence of mineralized nodules in cardiac ventricular fibroblasts by using von Kossa and alizarin red S staining, and a colorimetric method for calcium quantification, respectively.
Keywords: CalcificationMaterials and Reagents
- Cardiac ventricular fibroblasts isolated from neonatal Sprague-Dawley rats by enzymatic dissociation (Lee et al., 2013)
Note: Our protocol can be used with various cell types such as osteoblasts and vascular smooth muscle cells. - Dulbecco’s Modified Eagle’s Medium (DMEM) with high glucose and 4 mM L-glutamine (Hyclone, catalog number: SH 30243.01 )
- Fetal Bovine Serum (FBS) (Hyclone, catalog number: SH 30919.03 )
- Inorganic phosphate (Pi) (pH 7.4)
- DPBS without Ca2+ and Mg2+ (Hyclone, catalog number: SH 30028.02 )
- Trypsin/EDTA (Hyclone, catalog number: SH 30042.01 )
- 70% ethanol
- Distilled deionized water (DDW)
- 0.6 N HCl solution
- Lysis solution
- 0.1 N NaOH
- 0.1% sodium dodecyl sulphate (SDS)
- QuantiChrome Calcium Assay Kit (BioAssay Systems, catalog number: DICA-500 )
- Working reagent (refer to QuantiChrome Calcium Assay Kit manual for detail) (BioAssay Systems, catalog number: DICA-500) (see Reference 2)
- Bio-Rad DC protein assay kit (Bio-Rad Laboratories, catalog number: 500-0016 )
- Diluted protein standards (e.g., 0, 2, 4, 6, 8, 12, 16 and 20 mg/dl)
- 100 ng/ml receptor activator of NF-kB ligand (RANKL) (Sigma-Aldrich, catalog number: R0525 )
- 5% Aqueous silver nitrate solution (Sigma-Aldrich, catalog number: S7279 ) (see Recipes)
- 5% sodium thiosulfate (Sigma-Aldrich, catalog number: S7026 ) (see Recipes)
- 2% alizarin red S solution (Sigma-Aldrich, catalog number: A5533 ) (see Recipes)
- 1 M NaH2PO4 (pH 7.4) (Sigma-Aldrich, catalog number: S6566 ) (see Recipes)
Equipment
- 48-well plate
- 96-well plate
- Spectrophotometer or 96-well reader
- 37 °C, 5% CO2 cell culture incubator
- Inverted microscope
- Aspirator
- UV-visualizer
- High-watt lamp (60-100 watt)
Procedure
- Detection of calcium deposits
- Von Kossa Staining
- Cardiac ventricular fibroblast cells (2 x 104 cells/well in 48-well plates) are cultured in 500 μl of DMEM containing 10% FBS in the absence or presence of 3 mM inorganic phosphate (Pi; pH 7.4) for 6 days in 37 °C, 5% CO2 cell culture incubator for inducing mineralization. The media are freshly changed every 2 days.
Note: For inducing ectopic calcification in cardiac fibroblasts by high concentration of inorganic phosphate. - The cultured cells are rinsed twice with 250 μl DPBS and fixed in 250 μl of ice-cold 70% ethanol for 1 h at room temperature without any agitation.
- After fixation, DPBS and ethanol are removed and rinse with DDW carefully.
- After aspirating DDW on top of the fixed cells, add 250 μl of 5% silver nitrate solution and place the cell container in bright light such as sunlight, UV light or high-watt lamp until calcium deposits turns proper black (or dark brown).
- Rinse in 3 changes of 250 μl DDW briefly by gentle shaking.
- Rinse un-reacted silver with 250 μl of 5% sodium thiosulfate and keep for 5 min at room temperature (optional).
- Rinse in 3 changes of 250 μl DDW briefly by gentle shaking.
- Cardiac ventricular fibroblast cells (2 x 104 cells/well in 48-well plates) are cultured in 500 μl of DMEM containing 10% FBS in the absence or presence of 3 mM inorganic phosphate (Pi; pH 7.4) for 6 days in 37 °C, 5% CO2 cell culture incubator for inducing mineralization. The media are freshly changed every 2 days.
- Alizarin red S staining
- Fix the cultured cells with 250 μl of ice-cold 70% ethanol for 1 h at room temperature.
- After 3 changes of washing with 250 μl of DDW, stain the cells with 250 μl of 2% alizarin red S stain solution for 30 to 60 min at room temperature.
- Rinse in 2 changes with 250 μl of DDW.
- After removal of unincorporated excess dye with DDW, the mineralized nodules were stained as red spots.
- Von Kossa Staining
- Calcium quantification
Calcium content of the supernatants is determined colorimetrically with use of the QuantiChrome Calcium Assay Kit as described by manufacturer’s instructions. Briefly,- Cultured cells are washed twice with 250 μl of DPBS and decalcified with 250 μl of 0.6 N HCl for 12 h.
- Prepare working reagent by mixing equal volume of reagent A and B. Equilibrate to room temperature before use.
- Transfer 5 μl of diluted standards or samples into each wells of a clear bottom 96-well plate.
- Add 200 μl of working reagent and tap lightly to mix.
- After incubation for 3 min at room temperature, read the absorbance at 570-650 nm with 96-well reader.
- The remaining cells are washed three times with 250 μl of DPBS and solubilized in 200 μl of lysis solution containing 0.1 N NaOH and 0.1% sodium dodecyl sulphate (SDS) at room temperature for 5 min.
- The protein content is measured with a Bio-Rad DC protein assay kit.
- Calcium content is normalized to the total protein content of the whole cells (μg of Ca2+/mg protein of whole cells, see Figure 1).
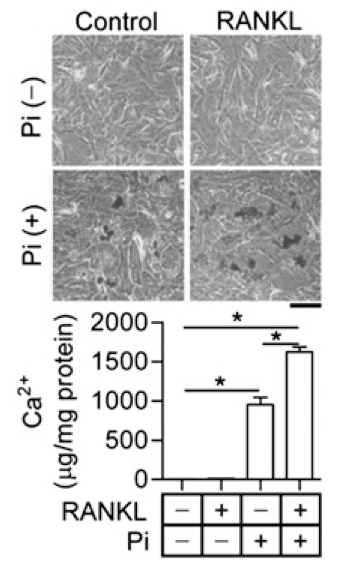
Figure 1. The synergic effect of RANKL on Pi-induced cardiac fibroblast calcification (Lee et al., 2013). Primary cardiac fibroblast cells were treated with 3 mM Pi in the absence or presence of 100 ng/ml RANKL for 3 d. Calcium content in cells was measured, and calcium deposits were visualized by von Kossa staining. A representative image is presented in the top panel. Scale bar, 100 μm (upper panel). Calcium contents were measured by QuantiChrome Calcium Assay kit (lower panel). Quantitative data are means ± SD (n = 3).
Recipes
- 5% Aqueous silver nitrate solution
Dissolve 5 g of silver nitrate in 100 ml DDW - 5% sodium thiosulfate
Dissolve 5 g of sodium thiosulfate in 100 ml DDW - 2% alizarin red S solution
Dissolve 2 g of alizarin red S (sodium alizarin sulphonate) in 100 ml DDW and the pH was adjusted to 4.1-4.3 using 0.5% ammonium hydroxide. - 1 M NaH2PO4 (pH 7.4)
Dissolve 1.2 g of NaH2PO4 in 10 ml DDW and the pH is adjusted to 7.4 using NaOH.
Acknowledgments
This protocol was adapted from the previously published report Lee et al. (2013) and the QuantiChrome Calcium Assay Kit manual.
References
- Lee, K., Kim, H., Park, H. S., Kim, K. J., Song, H., Shin, H. I., Kim, H. S., Seo, D., Kook, H., Ko, J. H. and Jeong, D. (2013). Targeting of the osteoclastogenic RANKL-RANK axis prevents osteoporotic bone loss and soft tissue calcification in coxsackievirus B3-infected mice. J Immunol 190(4): 1623-1630.
- QuantiChrome Calcium Assay Kit manual. http://www.bioassaysys.com/file_dir/DICA.pdf.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lee, K., Kwon, M. and Jeong, D. (2014). In vitro Biomineralization Assay. Bio-protocol 4(3): e1036. DOI: 10.21769/BioProtoc.1036.
Category
Immunology > Immune cell staining
Biochemistry > Other compound > Ion > Calcium
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












