- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
T Follicular Helper Cell Coculture Assay
Published: Vol 4, Iss 1, Jan 5, 2014 DOI: 10.21769/BioProtoc.1021 Views: 16699
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2663 Views

Primary Mouse Choroidal Endothelial Cell Culture
Qiuhua Yang [...] Yuqing Huo
Jun 20, 2025 2210 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3576 Views
Abstract
T follicular helper (Tfh) cells constitute a distinct subset of CD4+ T cells specialized in providing help to B cells in germinal centers. Phenotypically, Tfh cells are characterized by their high expression of the chemokine receptor CXCR5 that allows their migration into B cell follicles as well as high expression of PD-1, BTLA, the co-stimulatory molecules ICOS and SLAM and the transcription factors BCL6 and cMaf. Tfh cells are the main producers of IL-21 as well as other cytokines like IL-4 and IL-10 critical for B cell survival and differentiation. Tfh cells drive somatic hypermutation and the generation of long-lived memory B cells and plasma cells having an essential role in the development of protective immunity. Developing a coculture system to measure the effects of Tfh-cell mediated B cell help is of great interest to further our understanding of Tfh-B cell interaction and to allow for the manipulation of culture conditions to investigate the potential effect different microenvironment signals or ligand/receptor interactions could have on Tfh cell function.
Materials and Reagents
- Lymph node mononuclear cells (LMNCs)
- Benzonse® Nuclease (EMD Millipore, catalog number: 70664 )
- LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Life Technologies, catalog number: L34957 )
- Staphylococcal enterotoxin B (Toxin Technology Inc, catalog number: BT202 )
- RPMI 1640 Medium 1x with L-Glutamine (Fischer Scientific, catalog number: 10-040-CV )
- 100x Penicillin-Streptomycin solution (5000 units) (Life Technologies, catalog number: 15070063 )
- Fetal Bovine Serum (FBS) (Access Cell Culture)
- RPMI 1640 Medium 1x with L-Glutamine and no Phenol red (Gibco®, Life Technologies, catalog number: 11835-030 )
- HEPES solution (1 M) (Sigma-Aldrich, catalog number: H0887 )
- Antibodies:
- Complete media (see Recipes)
- Sorting buffer (see Recipes)
Equipment
- Centrifuge (SorvallTM LegendTM XTR, Thermo Fisher Scientific)
- 15 ml Falcon tube
- BD FACSAria II cell sorter (BD Biosciences)
- 37 °C 5% CO2 incubator
- 96-well sterile V-bottom plates (Thermo Fisher Scientific, catalog number: 249935 )
- 5 ml polystyrene round-bottom tubes with cell-strainer cap (BD FalconTM, catalog number: 352235 )
Procedure
- Thaw lymph node mononuclear cells (LMNCs) and wash once to remove all traces of DMSO carried from the freezing media. For information on lymph node samples and processing protocols see reference 1 (Cubas et al., 2013).
- Count the cells and resuspend at 1 x 107 cells/ml in complete media in a 15 ml Falcon tube.
- Treat the cells for 30 min in an incubator at 37 °C 5% CO2 by adding 25 U/ml of Benzonase® Nuclease to degrade all DNA/RNA and improve the viability of the cells.
- Pellet the cells in ice cold sorting buffer (containing HEPES) at 1,500 rpm for 5 min and resuspend in sorting buffer at a concentration of 5 x 107 cells/ml.
- Stain cells with a viability dye such as LIVE/DEAD® Fixable Aqua Dead Cell kit (Vivid) following the manufacturer’s instructions.
- Subsequently, proceed to stain the cells with the following antibodies: anti-CD3 (clone: HIT3a), anti-CD4 (clone: RPA-T4), anti-CD45RA (clone: IM2711U), anti-CXCR5 (clone: RF8B2) to gate on Tfh cells. Additionally and based on the population of B cells that you want to include in your coculture assay include B cell markers. For germinal center B cells you could use the following general markers: anti-CD19 (clone: HIB19), anti-IgD (clone: IA6-2), anti-CD38 (clone: HIT2).
- Stain the cells for 15 min on ice.
- Resuspend the cells in sorting buffer and spin at 1,500 rpm for 5 min.
- Remove the supernatant and resuspend at 3 x 107 cells/ml for sorting. Filter the cells by passing them through a 5 ml polystyrene round-bottom tube with cell-strainer cap.
- Proceed to cell sorting. Gate Tfh cells and germinal center (GC) B cells after excluding doublets and dead cells as: CD3+CD4+CD45RA-CXCR5++ (Figure 1) and CD3-CD19+CD38+IgD- (Figure 2) respectively.
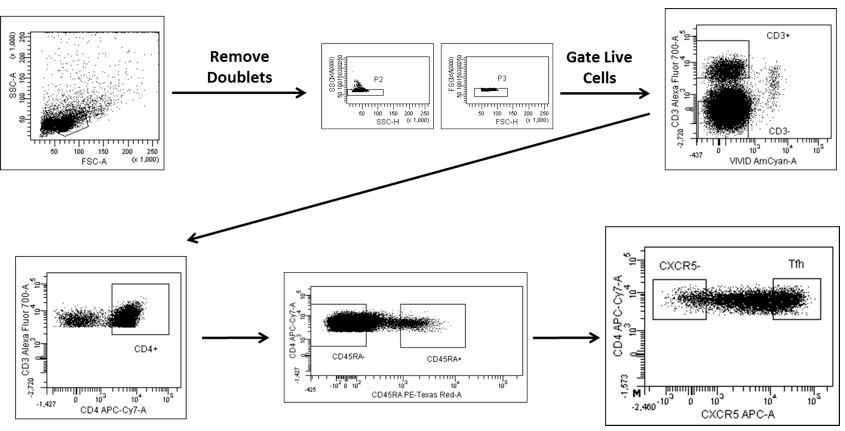
Figure 1. Gating strategy for sorting Tfh cells. Tfh cells are gated as CD3+CD4+CD45RA- CXCR5++ cells after excluding doublets and dead cells.
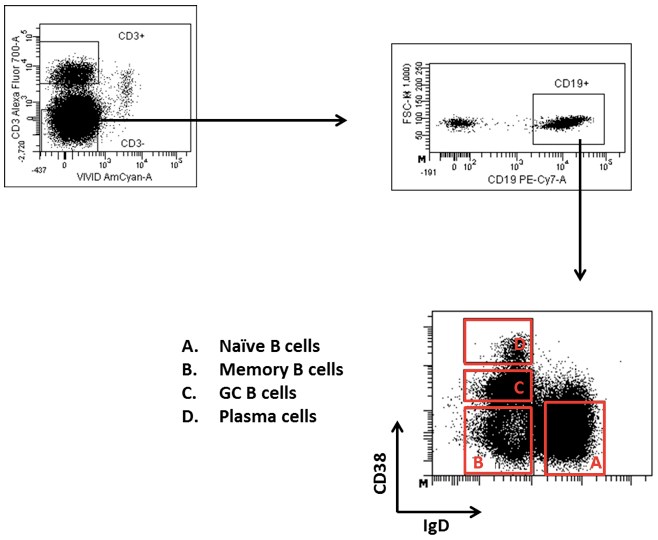
Figure 2. Gating strategy for sorting GC B cells. GC B cells are gated as CD3-CD19+IgD-CD38+ cells after excluding doublets and dead cells. The different B cell populations in the CD38-IgD dot plot are depicted.
- Once the cells are sorted, resuspend in complete media and centrifuge at 1,500 rpm for 5 min. Carefully remove the supernatant and make sure all the media is taken out. Resuspend the cells in either 250 μl or 500 μl of complete media (depending on number of sorted cells) and count the cells. It is highly recommended to have a post-sort check of the different cell populations sorted to confirm the cell purity.
- Resuspend the cells (both sorted Tfh cells and GC B cells) in complete media and spin down again at 1,200 rpm for 5 min. Remove the supernatant carefully and thoroughly and resuspend in an appropriate amount of complete media to have 1 x 105 cells in 50 μl (you can decide to resuspend at other concentrations but the coculture will have a final volume of 100 μl so you don’t want to have a volume larger than 50 μl for 1 x 105 cells since you are culturing both Tfh cells and GC B cells).
- Transfer 50 μl (1 x 105 cells) from both the sorted Tfh cells and GC B cells into a well of a 96-well V-bottom plate (1:1 ratio).
- Add 100 ng/ml of Staphylococcal enterotoxin B (SEB) into each well to mimic the antigen-specific interaction between T cells and B cells. Flick the plate carefully to mix and centrifuge at 900 rpm for 1 min.
- Incubate the cells at 37 °C 5% CO2 for 7 days or a different number of days based on the readout being measured. Testing the viability of the cells and readout measurements at later time points is recommended. Measuring the total levels of IgG in the coculture supernatant can be used as a measure of Tfh-mediated B cell help. Negative controls can include the incubation of GC B cells with naïve CD45RA+ cells or CD45RA-CXCR5- cells which do not provide B cell help. Cell characterization by flow cytometry can also be used to measure B cell activation and absolute numbers of live cells at the desired time point.
Recipes
- Complete media
RPMI 1640 (with L-Glutamine)
10% FBS
1x Pen/Strep
- Sorting buffer
RPMI 1640 (with L-Glutamine + no Phenol red)
2% FBS
1x Pen/Strep
25 mM HEPES
Acknowledgments
This protocol was first used in Cubas et al. (2013).
References
- Cubas, R. A., Mudd, J. C., Savoye, A. L., Perreau, M., van Grevenynghe, J., Metcalf, T., Connick, E., Meditz, A., Freeman, G. J., Abesada-Terk, G., Jr., Jacobson, J. M., Brooks, A. D., Crotty, S., Estes, J. D., Pantaleo, G., Lederman, M. M. and Haddad, E. K. (2013). Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19(4): 494-499.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cubas, R. A. and Haddad, E. K. (2014). T Follicular Helper Cell Coculture Assay. Bio-protocol 4(1): e1021. DOI: 10.21769/BioProtoc.1021.
Category
Immunology > Immune cell isolation > Lymphocyte
Cell Biology > Cell isolation and culture > Cell growth
Immunology > Immune cell function > Lymphocyte
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









