- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cell Surface Protein-protein Binding on COS-7 Cells
Published: Vol 4, Iss 1, Jan 5, 2014 DOI: 10.21769/BioProtoc.1020 Views: 11235
Reviewed by: Xuecai Ge

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measuring the Endocytic Recycling of Amyloid Precursor Protein (APP) in Neuro2a Cells
Florent Ubelmann [...] Claudia Guimas Almeida
Dec 5, 2017 8138 Views

Non-separate Mouse Sclerochoroid/RPE/Retina Staining and Whole Mount for the Integral Observation of Subretinal Layer
Sung-Jin Lee and Soo-Young Kim
Jan 5, 2021 4457 Views

Time-Lapse Into Immunofluorescence Imaging Using a Gridded Dish
Nick Lang [...] Andrew D. Stephens
Feb 20, 2026 50 Views
Abstract
Examination of interactions between a transmembrane protein and a soluble protein by pull-down or immunoprecipitation assays can be tricky and complicated due to the detergent extraction of membrane proteins during the lysate preparation step. The choice and concentration of detergents must be determined empirically and the procedure can be burdensome. Here, we describe a simplified binding assay by expressing the membrane protein of interest in COS-7 cells and applying detergent-free solutions containing an extracellular protein to be tested. The binding is then examined by immunocytochemistry.
Keywords: Membrane proteinMaterials and Reagents
- COS-7 cells (ATCC, catalog number: CRL-1651 TM)
- A mammalian expression plasmid encoding a membrane protein of interest
- A plasmid encoding an extracellular protein fused to the Fc portion of the human IgG [for example, pFUSE series by InvivoGen and pSXFc in Eshed et al. (2005) are options for cloning vectors to make this fusion construct]
- Dulbecco's modified Eagle Medium (DMEM), high glucose, no glutamine (Life Technologies, catalog number: 11960-044 )
- FetalClone III serum (Thermo Fisher Scientific, catalog number: SH30109.03 )
- 100x GlutaMAX-I (Life Technologies, catalog number: 35050-061 )
- Lipofectamine 2000 (Life Technologies, catalog number: 11668-019 )
- Dulbecco's phosphate-buffered saline (DPBS) (no calcium, no magnesium) (Life Technologies, catalog number: 14190-144 )
- Virus production serum-free medium (VP-SFM) (Life Technologies, catalog number: 11681-020 )
- 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: H4034 )
- Protein Concentrators (9K MWCO, 7 ml) (Thermo Fisher Scientific, catalog number: 87748 )
- Goat anti-human IgG, Fcγ fragment specific, conjugated with a fluorophore (e.g. Jackson ImmunoResearch Laboratories, catalog number: 109-545-098 for Fluorescein and 109-585-098 for Texas Red)
- COS-7 culture medium (see Recipes)
- 0.2 M PB (see Recipes)
- 4% PFA (see Recipes)
- PBS (see Recipes)
Equipment
- 6-well cell culture plates (Corning Incorporated, catalog number: 3516 )
- Glass coverslips (VWR International, catalog number: 48366-227 )
- 10-cm dish
- Centrifuge
- 37 °C/5% CO2 cell culture incubator
- A regular fluorescence microscope
Procedure
- Production of the medium containing the extracellular protein.
- Seed COS-7 cells in a 10-cm dish.
- Transfect the cells with the plasmid encoding the extracellular protein fused to the human IgG Fc (we use Lipofectamine 2000: 1 μg of DNA + 2 μl of Lipofectamine 2000 for one well in a 6-well plate, and follow the manufacturer’s instruction).
- One day after transfection, remove all the medium, wash the culture with 6 ml of DPBS twice and add 10 ml of VP-SFM containing 2x GlutaMAX-I.
- Two days later, collect all the medium and add 10 ml of fresh VP-SFM/2x GlutaMAX-I to the culture.
- Centrifuge the harvested medium at 3,000 x g for 3 min, collect the supernatant and neutralize it by adding to a final concentration 10 mM HEPES-Na+, pH 8.0.
- Repeat steps 1d and 1e.
- Pool two batches of the medium and concentrate with a protein concentrator to 1/10x the original volume. The concentrated medium can be stored at -80 °C(we usually store the concentrated medium in 1-ml aliquots. For our fusion proteins, the binding activity is still good even after 5 freeze-thaw cycles. If freezing and thawing are a concern, it is recommended to first test the binding activity from unfrozen preparations).
- Seed COS-7 cells in a 10-cm dish.
- Seed COS-7 cells directly into the 6-well plates containing glass coverslips (no treatment required) and the culture medium.
- Transfect the cells with the plasmid encoding the membrane protein.
- Two days later, thaw the concentrated medium containing the Fc fusion protein from step 1.
- For the binding assay on one coverslip of cells, take 100 μl of the concentrated medium and add the goat anti-human Fc antibodies conjugated with a fluorophore at a final concentration of 7.5 ng/ml. Incubate on ice for 30 min (cover with foil to protect the fluorophore from the light).
- Transfer the coverslips with cells from the original 6-well plate to a new empty 6-well plate and add the mixture from step 5 onto the coverslips carefully (the 100-μl mixture stays on the top of the coverslip.) Incubate at room temperature for 30 min (cover with foil).
- Remove the mixture of the concentrated medium and goat anti-human Fc antibodies and rinse with 2 ml of ice-cold DPBS three times.
- Fix the cells on coverslips and follow the regular immunostaining procedure. The fixation and staining conditions depend on the properties of the primary and secondary antibodies. For most of our antibodies, the following procedure is performed: (protect from light for the whole procedure).
- Add 2 ml of ice-cold 4% PFA per well and incubate for 20 min at 4 °C.
- Rinse with 2 ml of room-temperature PBS three times.
- The cells on the coverslips are ready for blocking/permeabilizing and immunostaining to visualize the expression of the membrane protein.
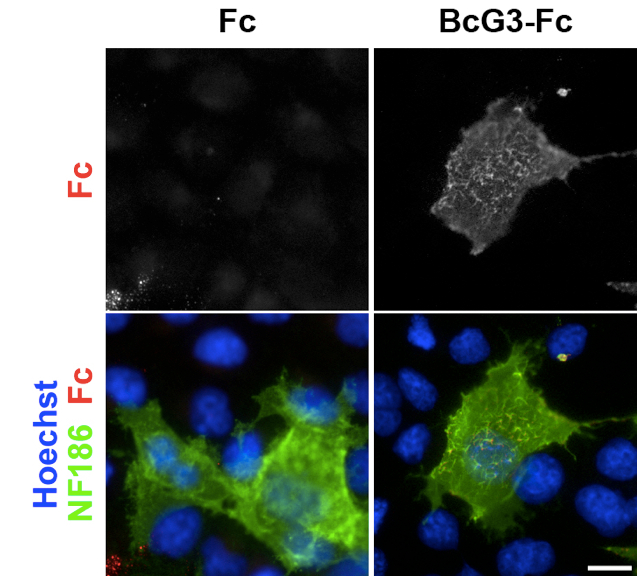
Figure 1. An example of the binding assay. COS-7 cells were transfected with an NF186-expressing plasmid and then incubated with a mixture of goat anti-human Fc antibodies and concentrated medium containing Fc or BcG3-Fc. The nuclei were visualized by Hoechst. Fc does not interact with NF186 and is the negative control. BcG3-Fc specifically interacts with NF186 and does not bind to untransfected cells. Scale bar = 16 μm.
- Add 2 ml of ice-cold 4% PFA per well and incubate for 20 min at 4 °C.
Recipes
- COS-7 culture medium (100 ml)
89 ml of DMEM
10 ml of FetalClone III
1 ml of 100x GlutaMAX-I
Stored at 4 °C
- 0.2 M PB (1L)
Dissolve 4.56 g of NaH2PO4 and 23.004 g of Na2HPO4 in 900 ml of ddH2O and adjust to 1,000 ml
Stored at room temperature
- 4% PFA (100 ml)
Dissolve 4 g of paraformaldehyde in 50 ml of 50-60 °C ddH2O by adding a few drops of 10 N NaOH
Add 50 ml of 0.2 M PB
Adjust pH to 7.2-7.4 by adding concentrated HCl
Good for 2 days if stored at 4 °C
- PBS
150 mM NaCl
10.4 mM sodium phosphate (pH 7.2)
Acknowledgments
This protocol was adapted from Eshed et al. (2005) and Susuki et al. (2013).
References
- Eshed, Y., Feinberg, K., Poliak, S., Sabanay, H., Sarig-Nadir, O., Spiegel, I., Bermingham Jr, J. R. and Peles, E. (2005). Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron 47(2): 215-229.
- Susuki, K., Chang, K.-J., Zollinger, D. R., Liu, Y., Ogawa, Y., Eshed-Eisenbach, Y., Dours-Zimmermann, M. T., Oses-Prieto, J. A., Burlingame, A. L. and Seidenbecher, C. I. (2013). Three mechanisms assemble central nervous system nodes of ranvier. Neuron 78(3): 469-482.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chang, K. and Rasband, M. N. (2014). Cell Surface Protein-protein Binding on COS-7 Cells. Bio-protocol 4(1): e1020. DOI: 10.21769/BioProtoc.1020.
Category
Biochemistry > Protein > Immunodetection > Immunostaining
Cell Biology > Cell structure > Cell surface
Molecular Biology > Protein > Protein-protein interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








