- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Potato Transformation
Published: Vol 4, Iss 1, Jan 5, 2014 DOI: 10.21769/BioProtoc.1017 Views: 19715
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Targeting Ultrastructural Events at the Graft Interface of Arabidopsis thaliana by A Correlative Light Electron Microscopy Approach
Clément Chambaud [...] Lysiane Brocard
Jan 20, 2023 2981 Views

Agrobacterium-mediated Genetic Transformation of Cotton and Regeneration via Somatic Embryogenesis
Alka Srivastava [...] Praveen C. Verma
May 20, 2023 4358 Views

A Novel Gene Stacking Method in Plant Transformation Utilizing Split Selectable Markers
Guoliang Yuan [...] Xiaohan Yang
Feb 20, 2025 1979 Views
Abstract
This is a protocol to produce stable transgenic potato plants (Solanum tuberosum cv. Désirée) by Agrobacterium-mediated genetic transformation, which is established based on a method described by (Jung et al., 2005) with some modifications. Agrobacterium tumefaciens strain LBA4404 carrying the desired construct is used to infect internodal explants to produce stable transgenic potato plants. Plantlet screening and molecular analyses are employed to confirm the expression of transgene in generated transgenic potato lines.
Materials and Reagents
- Potato (S. tuberosum cv. Désirée) tissue culture plantlets
- 70% ethanol
- Bleach (6% sodium hypochlorite)
- Tween 20
- Acetosyringone (Fisher Scientific, catalog number: AC11554 )
- Timentin (PhytoTechnology Laboratories®, catalog number: T869 )
- Selective antibiotics
- Mannitol (Fisher Scientific, catalog number: M120 )
- Glycine
- Nicotinic acid (Sigma-Aldrich, catalog number: N0761 )
- Pyridoxine HCl (Sigma-Aldrich, catalog number: P6280 )
- Thiamine HCl (Sigma-Aldrich, catalog number: T1270 )
- Folic acid (Fisher Scientific, catalog number: BP2519 )
- Biotin (Fisher Scientific, catalog number: BP232 )
- MS salt (Caisson Laboratories, catalog number: MSP01-50LT )
- Inositol (Fisher Scientific, catalog number: AC122261000 )
- Sucrose
- 6-benzylamino purine (BAP) (Sigma-Aldrich, catalog number: B3408 )
- 1-naphthalene-acetic acid (NAA) (Sigma-Aldrich, catalog number: N0640 )
- 3-indoleacetic acid (IAA) (Sigma-Aldrich, catalog number: I2886 )
- Trans-zeatin-riboside (Sigma-Aldrich, catalog number: Z0876 )
- YM medium (see Recipes)
- MSVI vitamins (see Recipes)
- JHMS vitamins (see Recipes)
- 3R vitamins (see Recipes)
- CIM medium (see Recipes)
- 3C5ZR plates with selective agent (see Recipes)
- Propagation medium (see Recipes)
- TPS buffer (see Recipes)
Equipment
- Sterile magenta boxes (sterilized by autoclaving)
- Sterile petri dishes (Fisher scientific, catalog number: FB0875712 )
- Sterile forceps and scalpel (sterilized by heat treatment using a Bunsen burner)
- Sterile glass tubes with caps (sterilized by autoclaving) (tubes, Fisher Scientific, catalog number: 14-961-34 ; caps, Fisher Scientific, catalog number: 14-957-91D )
- Sterile inoculating loop (Fisher Scientific, catalog number: 22-363-604 )
- Tissue grinders
- Glass culture tubes (sterilized by autoclaving)
- 3M Micropore tape (Fisher Scientific, catalog number: 1530-0 and 1530-1 )
- Agrobacterium glycerol stock
- 50 ml conical centrifuge tubes
- Tissue culture biosafety cabinet
- Incubator shaker (New Brunswick Scientific, model: C24KC )
- Centrifuge
- S. tuberosum growth chambers (we use several types of growth chambers such as I-66LLVL from Percival)
- Hot block or water bath
- PCR thermal cycler (Bio-Rad Laboratories, model: C1000 touch)
Procedure
- Generation and propagation of potato tissue culture plantlets (performed under sterile conditions)
- Excise sprouts (at least 2.5 to 4 cm long) from potato tubers or stem segments (containing at least one internode) from plants and surface sterilize these plant pieces:
- Wash with 70% ethanol for 1-2 min.
- Use a sterile forceps to transfer plant pieces to 10% bleach containing trace amount of Tween 20 (1 μl/ml) and soak for 10-12 min.
- Transfer plant pieces to 70% ethanol and rinse for 1-2 min.
- Wash with plenty of sterile distilled water to remove any trace of ethanol.
- Wash with 70% ethanol for 1-2 min.
- Trim the sterilized plant pieces with sterile scalpel to remove damaged cell layers and culture each plant piece on propagation medium in a glass tube at 24 °C under a 16-h/8-h light/dark cycle. Plantlets originated from the sterilized plant pieces are generally established for approximately four to six weeks.
- Cut approximately 1 cm-long nodal stem segments from plantlets (Figure 1), and push firmly into a magenta box or glass tube containing propagation medium, leaving the top part of the stem exposed. Put six segments per box or one segment per glass tube, and seal the box cover or tube cap with micropore tape. Around twenty four plantlets are suitable for transformation of one construct.
- Cultivate in a growth incubator at 24 °C under a 16-h/8-h light/dark cycle for four to six weeks.
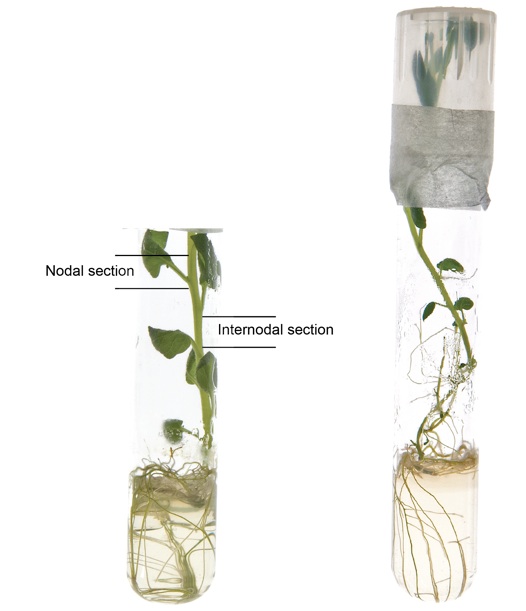
Figure 1. Potato plantlet growing in the propagation medium. Nodal and internodal sections are indicated as shown.
- Excise sprouts (at least 2.5 to 4 cm long) from potato tubers or stem segments (containing at least one internode) from plants and surface sterilize these plant pieces:
- Agrobacterium preparation
- Four days prior to infection, streak out Agrobacterium tumefaciens LBA4404, carrying the desired construct (transgene), on an YM plate with appropriate antibiotics. Incubate the plate at 28 °C for three days.
- The day before infection, inoculate 10 ml of YM with Agrobacterium colonies and culture at 28 °C on an incubator shaker till OD00 reaches 0.7 (usually need overnight culturing).
- On the day of inoculation, centrifuge the Agrobacterium culture at 9,000 x g for 15 min at room temperature and re-suspend the pellet in 10 ml CIM liquid medium.
- Add 50 μl of 0.074 M acetosyringone to the Agrobacterium suspension (the final concentration of acetosyringone is 0.37 mM). Use within one hour, as cells will begin to aggregate.
- Four days prior to infection, streak out Agrobacterium tumefaciens LBA4404, carrying the desired construct (transgene), on an YM plate with appropriate antibiotics. Incubate the plate at 28 °C for three days.
- Potato transformation (performed under sterile conditions)
- On the day of infection, cut internodal stem sections (Figure 1) from four to six weeks plantlets into about 1 cm pieces and transfer to CIM plates.
- Incubate explants with 10 ml Agrobacterium inoculum for 20 min, occasionally swirling the plates. We usually use 100 stem pieces per construct.
- Use sterile forceps to transfer stem pieces from the bacterial culture to new CIM media plates. Label plates, seal with micropore tape, and place them back to the 24 °C incubator in the dark.
- After three days, transfer stem pieces to 3C5ZR medium plates with appropriate antibiotics and 500 μg/ml timentin. Timentin is used to inhibit the growth of A. tumefaciens LBA4404.
- Place the plates in the growth incubator (24 °C, 16-h/8-h light/dark cycle) and transfer the stem pieces to fresh 3C5ZR medium plates every seven to ten days, until plantlets emerge (Figure 2).
- When plantlets are at least half a centimeter long, cut them from explants and transfer to culture tubes containing 10 ml of propagation medium with the appropriate antibiotics and timentin at 100 μg/ml. Sterile inoculating loops can be used to gently push the base of the plantlet into the medium. Label and seal the tubes with micropore tape.
- Grow plants for 3 - 4 weeks until roots are established. Discard any plants that do not grow roots within one month, as they are not transgenic.
- Cut and re-propagate the transgenic plants as needed.

Figure 2. Potato internodal sections cultivated on the 3C5ZR medium. Plantlets (indicated) are emerging from the infected nodal or internodal sections.
- On the day of infection, cut internodal stem sections (Figure 1) from four to six weeks plantlets into about 1 cm pieces and transfer to CIM plates.
- PCR screening
- Cut approximately one-half to one square centimeter of leaf tissue to a 1.5 ml centrifuge tube.
- Add 200 μl TPS to each tube.
- Grind the tissue in TPS with a tissue grinder to obtain a uniform slurry.
- Incubate samples at 75 °C for 20 min.
- Centrifuge at 13,400 x g for 10 min.
- Transfer supernatant to a new tube and add an equal volume of isopropanol to each tube. Mix tubes by inversion.
- Centrifuge at 13,400 x g for 10 min. DNA pellet should be visible at this point.
- Remove supernatant and wash pellet with 70% ethanol. Centrifuge at 13,400 x g for 5 min.
- Remove supernatant, air-dry pellet and resuspend pellet in 30 μl of sterile distilled water or TE containing RNaseA (100 μg/ml).
- Use 1 μl of DNA as template for PCR screening of transformants.
- Typical PCR program:
First step: 95 °C for 3 min
Second step: 94 °C for 30 sec, 58 °C for 30 sec, 72 °C for 1 min x 30 cycles
Third step: 72 °C for 5 min
- Cut approximately one-half to one square centimeter of leaf tissue to a 1.5 ml centrifuge tube.
Recipes
The following media are prepared and stored at room temperature. The poured medium plates containing antibiotics can be stored at 4 °C for up to one month. Vitamins can be prepared, aliquoted and stored at -20 °C.
- YM Medium (1 L)
0.4 g yeast extract
10 g mannitol*
0.1 g NaCl
0.2 g MgSO4.7H2O
0.5 g KH2PO4
10 g Agar (for plates)
Autoclave
*Make and autoclave separately, make 10 g/100 ml and use 100 ml/L - MSVI vitamins
2 mg/ml glycine
0.5 mg/ml nicotinic acid
0.5 mg/ml pyridoxine HCl
0.4 mg/ml thiamine HCl - JHMS vitamins
0.4 mg/ml folic acid
50 μg/ml biotin - 3R vitamins
1 mg/ml thiamine HCl
0.5 mg/ml nicotinic acid
0.5 mg/ml pyridoxine HCl - CIM medium (1 L)
4.3 g MS salt
1 ml MSVI vitamins
1 ml JHMS vitamins
0.1 g inositol
30 g sucrose
1 mg 6-benzylamino purine (BAP)
2 mg 1-naphthalene-acetic acid (NAA)
10 g agar (for plates)
Adjust pH to 5.6 with KOH and autoclave - 3C5ZR medium (1 L)
4.3 g MS salt
1 ml 3R vitamins
0.1 g inositol
30 g sucrose
10 g agar (for plates)
Adjust pH to 5.9 with KOH and autoclave
0.5 mg 3-indoleacetic acid (IAA) *
3 mg trans-zeatin-riboside *
500 mg Timentin*
Selection agent **
* Add after sterilization
** Use appropriate selection agent and concentration for construct - Propagation medium (1 L)
4.3 g MS salt
0.17 g NaH2PO4.H2O
0.1 g inositol
0.4 mg thiamine HCl
30 g sucrose
2.5 g gelrite or 10 g agar
Adjust pH to 6.0 with KOH and autoclave - TPS buffer
100 mM Tris HCl (pH 8.0)
100 mM EDTA (pH 8.0)
1 M KCl
Acknowledgments
This work was supported by funding from USDA-ARS.
References
- Cheng, Z. M., Schnurr, J. A. and Kapaun J. A. (1998). Timentin as an alternative antibiotic for suppression of Agrobacterium tumefaciens in genetic transformation. Plant Cell Rep 17: 646-649.
- Chronis, D., Chen, S., Lu, S., Hewezi, T., Carpenter, S. C., Loria, R., Baum, T. J. and Wang, X. (2013). A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant J 74(2): 185-196.
- Jung, C. S., Griffiths, H. M., De Jong, D. M., Cheng, S., Bodis, M. and De Jong, W. S. (2005). The potato P locus codes for flavonoid 3',5'-hydroxylase. Theor Appl Genet 110(2): 269-275.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chronis, D., Chen, S., Lang, P., Tran, T., Thurston, D. and Wang, X. (2014). Potato Transformation. Bio-protocol 4(1): e1017. DOI: 10.21769/BioProtoc.1017.
Category
Plant Science > Plant transformation > Agrobacterium
Molecular Biology > DNA > Transformation
Plant Science > Plant physiology > Tissue analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link













