- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Nematode Infection on Potato Plant
Published: Vol 4, Iss 1, Jan 5, 2014 DOI: 10.21769/BioProtoc.1016 Views: 15050
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Infection Assay of Cyst Nematodes on Arabidopsis Roots
Holger Bohlmann and Krzysztof Wieczorek
Sep 20, 2015 12402 Views
Root-knot Nematode Penetration and Sclareol Nematicidal Activity Assays
Taketo Fujimoto [...] Shigemi Seo
Jun 20, 2016 11183 Views

Culturing Bacteria from Caenorhabditis elegans Gut to Assess Colonization Proficiency
Facundo Rodriguez Ayala [...] Roberto Grau
Jun 20, 2017 16298 Views
Abstract
Potato cyst nematodes (PCNs; Globodera rostochiensis and G. pallida) are devastating pests that infect potato root. We describe an in vitro assay for PCN infection on potato plantlet in tissue culture. This method is useful for studying nematode parasitism on potato and for investigating responses of potato clones/lines to PCN infection.
Keywords: Potato cyst nematodeMaterials and Reagents
- Potato plant (Solanum tuberosum cv. Désirée)
- Potato cyst nematode (G. rostochiensis) cysts
- Sodium azide (RICCA Chemical, catalog number: 7144-16 )
- Mercuric chloride (RICCA Chemical, catalog number: 4650-16 )
- Sterile distilled water
- Gentamycin (Fisher Scientific, catalog number: 61398-0010 )
- Nystatin (Sigma-Aldrich, catalog number: N-3503 )
- Agarose (Fisher Scientific, catalog number: BP160-100 )
- Timentin (PhytoTechnology Laboratories®, catalog number: T869 )
- Micropore tape (Fisher Scientific, catalog number: 19-027-761 )
- Sterilization solution (0.004% mercuric chloride/0.004% sodium azide)
- Hoagland's solution (Sigma-Aldrich, catalog number: H2395 )
- Amberlite XAD-4 resin (Fisher Scientific, catalog number: AC20223 )
- MS salt (Caisson Laboratories, catalog number: MSP01-50LT )
- Inositol (Fisher Scientific, catalog number: AC122261000 )
- Thiamine HCl (Sigma-Aldrich, catalog number: T1270 )
- Gelrite (Fisher Scientific, catalog number: CAS 71010-52-1 )
- Potato root diffusate (PRD) (see Recipes)
- Propagation medium (see Recipes)
- 0.1% agarose (see Recipes)
Equipment
- Sieves No. 60 (250 μm), No. 200 (75 μm) and No. 500 (25 μm) (Endecotts, catalog number: 683927 , 681837 , 691131 )
- Sterile nematode egg hatching chamber (stainless steel metal pan cover and collection pan may be purchased from Vollrath Products, size six inches; the metal screened pan cover with wire-screen in the central area as shown in Figure 3 was custom made)
- Sterile 6-well plates (Greiner Bio-One GmbH, catalog number: 657185 )
- Stirplate
- Sterile forceps and scalpel
- Growth incubator
- 30-μm mesh (Small Parts, catalog number: 7050-1220-000-12 )
- Tissue culture biosafety cabinet
- Centrifuge
- Incubator shaker (New Brunswick Scientific, model: C24KC )
- S. tuberosum growth chambers (we use several types of growth chambers such as I-66LLVL from Percival)
- Rubber stopper (size no. 1) (Fisher Scientific, catalog number: 14-130C )
- Beaker
- Aluminum foil
- Dissecting microscope
Procedure
- Plant growth
- In a sterile environment, cut nodal stem segments (approximately 1 cm long) (Figure 1), and push the stem piece firmly into the 6-well plate containing 6 to 7 ml of propagation medium per well with timentin (100 μg/ml), leaving the top part of the stem exposed (Figure 2).
- Label plates and seal them with micropore tape.
- Cultivate in a growth incubator at 24 °C under a 16-h/8-h light/dark cycle for two weeks until roots have emerged for several days.

Figure 1. Potato plantlet growing in the propagation medium. Nodal section is indicated as shown.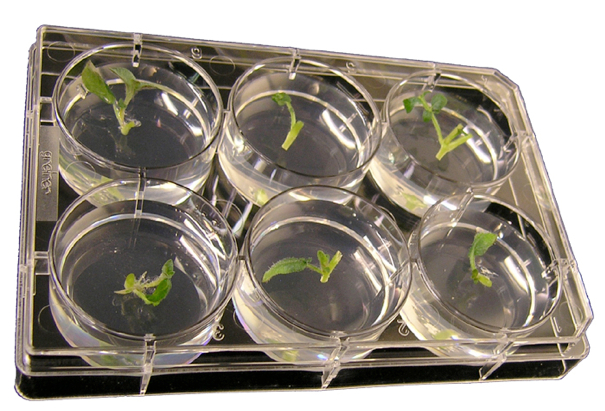
Figure 2. 6-well plate with potato nodal segments embedded in propagation medium
- In a sterile environment, cut nodal stem segments (approximately 1 cm long) (Figure 1), and push the stem piece firmly into the 6-well plate containing 6 to 7 ml of propagation medium per well with timentin (100 μg/ml), leaving the top part of the stem exposed (Figure 2).
- Nematode egg hatching and sterilization (all the following steps are performed at room temperature)
- One week before inoculation, soak nematode cysts at room temperature in a small (20-30 ml) beaker covered loosely with aluminum foil and check daily to ensure that cysts are fully soaked in water during the 7-day hydration period.
- Crush the cysts using the following method:
- Nest the sieves with the No. 60 sieve on top, No. 200 sieve in the middle and the No. 500 sieve on the bottom.
- Pour the cysts from their beaker to the sieve stack and rinse out the beaker with water to collect the remaining cysts.
- With a rubber stopper, grind the cysts through the No. 60 sieve using a circular motion, washing frequently with tap water to keep cysts off the sides. Grind until no more cyst pieces will go through the screen.
- Wash the No. 60 sieve thoroughly with water.
- Remove the top sieve and discard the contents. Wash the No. 200 sieve thoroughly with water and remove it. Nematode eggs are collected on the No. 500 sieve.
- Use a water bottle to rinse eggs from the No. 500 sieve into a clean 250 ml glass beaker.
- Nest the sieves with the No. 60 sieve on top, No. 200 sieve in the middle and the No. 500 sieve on the bottom.
- Add sodium azide solution (caution: TOXIC! Wear nitrile gloves) to beaker containing nematode eggs and make up to 100 ml with water. The egg suspension should have a final concentration of 0.02% sodium azide.
- Stir the egg suspension for 20 min on a stirplate.
- Pour the egg suspension back onto the previously used No. 500 sieve and rinse the eggs well with constant flow of tap water for about two min to remove all traces of sodium azide.
- Set up the hatching chamber (Figure 3). Nest a large mesh screen pan on a solid pan and place a 30-μm mesh on the screen pan. Rinse the nematode eggs from the No. 500 sieve onto the 30-μm mesh carefully using PRD containing gentamycin (1.5 mg/ml) and nystatin (0.1 mg/ml) antibiotics. Make sure to add enough PRD with antibiotics into the hatching chamber to cover the 30-μm mesh. Cover with an inverted solid pan and place in a tray. All the pans and the 30-μm mesh must be autoclaved before use.
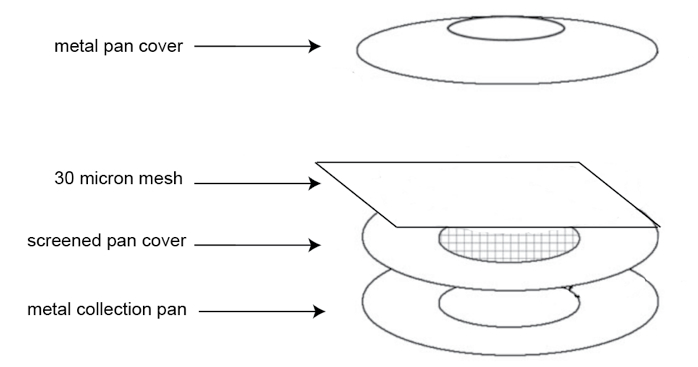
Figure 3. Nematode egg hatching chamber assembly - Allow eggs to hatch at room temperature for 3-4 days, making sure there is enough PRD (with appropriate antibiotics).
- Pour hatched juveniles (J2s) (Figure 4) into a small glass beaker several hours before using to let the worms settle down at the bottom of the beaker.
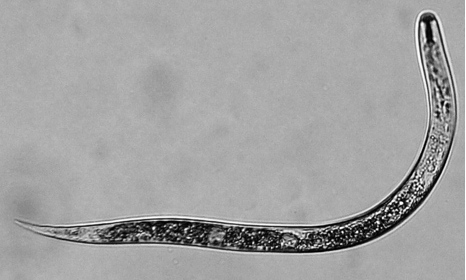
Figure 4. Hatched nematode juvenile (J2) - Check the J2 suspension under the dissecting scope for signs of heavy bacterial or fungal contamination. If heavily contaminated, do not use for inoculation work.
- Take out PRD as much as possible (without disturbing the settled J2s on the bottom) using a glass pipette and spin down the J2s at 13,400 x g for 2 min in 1.5 ml low retention centrifuge tubes. Resuspend J2s in 1 ml sterile water.
- Take counts of J2s by making 1:10 dilution and count J2s in five 10-μl drops. Calculate the total number of J2s in 1 ml according to the average number of J2s in a 10-μl drop, and spin down the amount of J2s needed for inoculation at 13,400 x g for 2 min (spin down more J2s than needed for infection, since some nematodes will die after sterilization). Proceed with sterilization.
- Sterilization
- Add 1 ml of diluted sterilization solution (0.004% mercuric chloride/0.004% sodium azide; Caution: TOXIC! Wear nitrile gloves) to each tube containing J2s. Close tubes and incubate on a rocking shaker for 10 min.
- Spin down the J2s at 13,400 x g for 2 min. Remove sterilization solution and discard in a special waste container (solutions containing mercury must not be discarded in a sink).
- Wash J2s with 1 ml sterile distilled water, incubate on the rocking shaker for 5 min and then centrifuge to remove water. Repeat washing steps for three more times.
- Resuspend J2s in 1 ml of sterile distilled water and take out 10 μl suspension to count. Calculate the volume needed to obtain the desired number of J2s for infection.
- Add 1 ml of diluted sterilization solution (0.004% mercuric chloride/0.004% sodium azide; Caution: TOXIC! Wear nitrile gloves) to each tube containing J2s. Close tubes and incubate on a rocking shaker for 10 min.
- Centrifuge to spin down the J2s and remove water as much as possible. Add calculated volume of 0.1% agarose to get the desired density of J2s for infection (recommend to use 1 - 3 J2 per μl).
- One week before inoculation, soak nematode cysts at room temperature in a small (20-30 ml) beaker covered loosely with aluminum foil and check daily to ensure that cysts are fully soaked in water during the 7-day hydration period.
- Infection of S. tuberosum with potato cyst nematode G. rostochiensis
- Cut off the tops of the potato plantlets in the 6-well plates using a sterile technique.
- Inoculate each potato plantlet with 100 - 200 nematode J2s depending on experiments. Pipette out a desired volume of J2 agarose suspension and punch the pipet tip into the medium and release the nematode suspension around the root tips (perform the inoculation within half an hour after J2s are suspended in 0.1% agarose since long suspension in this agarose solution can reduce nematode infectivity).
- Seal with micropore tape and return plates in the growth chamber (24 °C, dark condition).
- Monitor the development of G. rostochiensis on potato plantlet roots each week for the next four to five weeks. After two weeks of infection nematodes at J3 stage should be visible.
- Count nematode females four to five weeks post infection
- Take out each plantlet (including medium) and put on the lid of a petri dish (100 mm in diameter). Cut off the top of the plantlet and just keep the roots, add several drops of water on the plate, cover with another half of the petri dish on top. Gently press the top petri dish until the medium and the roots fully expanded on the bottom petri dish (Figure 5).
- Count the females on this plate under a dissecting microscope.
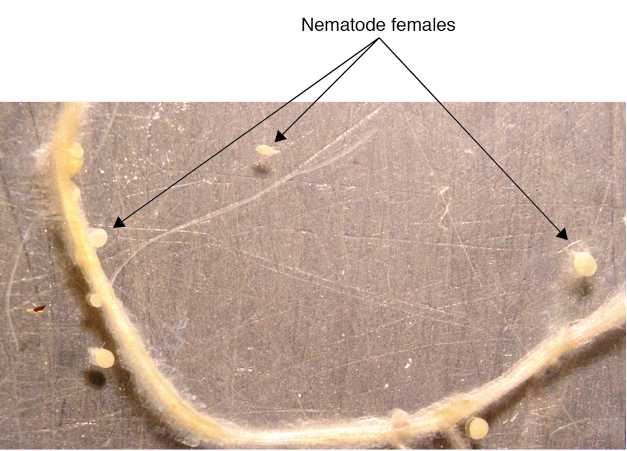
Figure 5. Nematode females formed on a potato root (some were detached from the root due to handling)
- Cut off the tops of the potato plantlets in the 6-well plates using a sterile technique.
Recipes
- Potato root diffusate (PRD)
Potatoes (tuber pieces with sprouts) are planted in 4 L inverted amber glass bottles filled with perlite and fitted at the bottom with tubing, grow within a greenhouse. Plants were fertilized with 1/2 strength Hoagland's solution and kept moist throughout the growing season. At weekly intervals, starting at three to ten weeks post planting, pots were filled with distilled water, let stand for 1 h, and then the water containing root diffusate was drained and collected. This material was passed through a column bed of Amberlite XAD-4 resin, column rinsed with 500 ml water, and then eluted with 500 ml methanol. The methanol elutant was rotor vaporated to a minimal volume (typically 2 ml), transferred to a vial, frozen with liquid nitrogen, and placed into a -80 °C freezer. Use 1:800 dilution with sterile distilled water for nematode egg hatching. - Propagation medium (1 L)
4.3 g MS salt
0.17 g NaH2PO4.H2O
0.10 g inositol
0.4 mg thiamine HCl
30 g sucrose
2.5 g gelrite
Adjust pH to 6.0 with KOH and autoclave - 0.1% Agarose
0.1g agarose in 100 ml distilled water
Autoclave and store at room temperature
Acknowledgments
This work was supported by funding from USDA-ARS.
References
- Baum, T. J., Wubben, M. J., Hardyy, K. A., Su, H. and Rodermel, S. R. (2000). A screen for Arabidopsis thaliana mutants with altered susceptibility to Heterodera schachtii. J Nematol 32(2): 166-173.
- Brodie, B. B. (1996). Effect of initial nematode density on managing Globodera rostochiensis with resistant cultivars and nonhosts. J Nematol 28: 510–519.
- Brodie, B. B., Plaisted, R. L. and de Scurrah, M. M. (1991). The incorporation of resistance to Globodera pallida into Solanum tuberosum germplasm adapted to North America. Am Potato J 68: 1-11.
- Chronis, D., Chen, S., Lu, S., Hewezi, T., Carpenter, S. C., Loria, R., Baum, T. J. and Wang, X. (2013). A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant J 74(2): 185-196.
- Clarke A. J. and Perry R. N. (1977). Hatching of cyst-nematodes. Nematologica 23: 350-368.
- Lu, S. W., Tian, D., Borchardt-Wier, H. B. and Wang, X. (2008). Alternative splicing: a novel mechanism of regulation identified in the chorismate mutase gene of the potato cyst nematode Globodera rostochiensis. Mol Biochem Parasitol 162(1): 1-15.
- Sijmons, P. C., Grundler, F. M., Mende, N., Burrows, P. R. and Wyss, U. (1991). Arabidopsis thaliana as a new model host for plant‐parasitic nematodes. Plant J 1(2): 245-254.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chronis, D., Chen, S., Lang, P., Tran, T., Thurston, D. and Wang, X. (2014). In vitro Nematode Infection on Potato Plant. Bio-protocol 4(1): e1016. DOI: 10.21769/BioProtoc.1016.
Category
Plant Science > Plant immunity > Disease bioassay
Cell Biology > Cell isolation and culture > Cell growth
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link














