- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Autophagy Assays (LC3B immunofluorescence, LC3B western blot, acridine orange assay)
Published: Vol 3, Iss 24, Dec 20, 2013 DOI: 10.21769/BioProtoc.1012 Views: 28813
Reviewed by: Lin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurement of CD74 N-terminal Fragment Accumulation in Cells Treated with SPPL2a Inhibitor
Rubén Martínez-Barricarte [...] Jean-Laurent Casanova
Jun 5, 2019 6981 Views

Capillary Nano-immunoassay for Quantification of Proteins from CD138-purified Myeloma Cells
Irena Misiewicz-Krzeminska [...] Norma C. Gutiérrez
Jun 20, 2019 6854 Views

Far-western Blotting Detection of the Binding of Insulin Receptor Substrate to the Insulin Receptor
Jinghua Peng [...] Ling He
Feb 20, 2023 2492 Views
Abstract
Autophagy is a dynamic cellular event that is involved in the degradation of long lived proteins and organelles in cells. Biochemical methods such as western blot to measure autophagic proteins have been increasingly used in autophagy studies because it is convenient and objective. Among them, the total amount of Microtubule-associated protein light chain 3 (LC3), the mammalian homologue of the autophagy-related Atg8 in yeast, is a very useful and most commonly used tool in autophagy studies. Other methods such as electron microscopy and immunofluorescence are also available to measure autophagy. The following protocol describes three simple and commonly used protocols for measuring autophagy in cells: LC3B immunofluorescence, western blot and acridine orange assay. Although these three methods are frequently used to provide basic information about autophagy, people should keep in mind that they are not enough to give the exact details about the autophagy flux due to the complexity of this dynamic process. In addition, acridine orange assay is only a supplementary method to detect autophagy because it also has high affinity to other organelles such as lysosomes. For further investigation about a compound’s effect on autophagy flux, additional and more complicated assays are recommended. Protocols here provide a starting point for people to get a snapshot of whether a compound can affect autophagy in tissue culture cells.
Keywords: AutophagyMaterials and Reagents
- 70% ethanol
- DMEM or other medium for tissue-culture cells
- Bovine Serum Albumin (BSA) (Sigma-Aldrich)
- Formaldehyde
- 100% Methanol
- PBS
- Rabbit antiLC3B antibody (LC3B (D11) XP® Rabbit mAb) (Cell Signaling Technology, catalog number: 3868 )
- HRP-conjugated secondary antibody
- Fluorescently labeled anti-rabbit secondary antibody (Molecular Probes, Life Technologies, catalog number: A-11008 )
- ProLong® Gold Antifade Reagent with DAPI (Life Technologies, catalog number: P36935 )
- M-PER lysis buffer (Pierce Antibodies, catalog number: 78501 )
- 5% Non-fat dry milk
- cOmplete ULTRA Protease inhibitor cocktail tablets (Roche Diagnostics, catalog number: 0 5892970001 )
- PhosSTOP phosphatase inhibitor cocktail tablets (Roche Diagnostics, catalog number: 0 4906837001 )
- Sample buffer (Bio-Rad Laboratories, catalog number: 161-0737 )
- Enhanced chemiluminescence (ECL)
- Acridine orange (Sigma-Aldrich, catalog number: A8097 )
- 4-15% Mini-PROTEAN TGX Precast Gel (Bio-Rad Laboratories, catalog number: 456-1085 )
- E64d/pepstatin A (Sigma-Aldrich, catalog number: E8640/P5318 )
- TBS (see Recipes)
- TBS-Tween (see Recipes)
- AbDil-Tw (see Recipes)
- TBS-Tx (see Recipes)
- AbDil-Tx (see Recipes)
- Running buffer (see Recipes)
- Transfer buffer (see Recipes)
Equipment
- 24-well plate
- 12-well plate (or 6-well plate)
- 1.5 ml tube
- Round microscope coverslips (Fisher Scientific, catalog number: 12-545-81 ) (these coverslips are ready to use and do not require extra coating procedure)
- Slides
- Tweezer with fine tips
- Transfer pipette
- Humid chamber (can be homemade from a 15 cm plate, parafilm, foil and wet kimwipes)
- Vacuum pump with a liquid bottle and pipe attached to remove washing solution
- Fluorescent microscope
- Western Blotting apparatus (SDS-PAGE running cassette, power supply, shaker, transfer cassette, PVDF membrane, etc.)
- Tissue culture apparatus (CO2 incubator, tissue culture hood, etc.)
Software
- MetaMorph
Procedure
- LC3B immunofluorescence
- Place one round coverslip per well in 24-well plate and add around 0.5 ml 70% ethanol for 1 min to sterilize the coverslip. Aspirate ethanol and let it dry for 5-10 min. Tissue cultured cells were plated on 24-well plate one day before experiment to let them attach to coverslips. [For cells to be plated on the coverslips, it depends on the time of drug treatment for your experiment so that the final cell confluency will be optimal for observation (80-90%)].
- On the day of experiment, treated with different concentrations of small molecule compounds for desired time.
- Fix the cell by replace with 0.5 ml cold 100% methanol (prestored in -20 °C) and fix for 5 min. Since the cold methonal is prestored in -20 °C and the fixation time is short, this step can be performed at RT on the bench. Cells can also be fixed in PBS + 4% formaldehyde at room temperature for 20-30 min followed by permeabilization with TBS-Tx for 20 min.
- Wash coverslips with 0.5 ml PBS once and replace with 0.5 ml AbDil-Tx to block. The block step can range from room temperature 30 min to 4 °C overnight. For example, people can use RT 30 min, 1 h, 2 h or 4 °C 2 h to overnight.
- Prepare primary antibody. LC3B antibody from cell signaling can be diluted at 1/200-1/500 in AbDil-Tx. Usually one coverslip need 30-100 μl.
- Transfer coverslips to the humid chamber. It can be made from a 15 cm round tissue culture plate. Cut a piece of square or rectangle parafilm that can fit the bottom of the plate. Use finger and tweezer to press down to make the parafilm adhere tightly to the bottom of the plate. The coverslips can then sit on the parafilm with liquid on top. Use a sheet of foil that can cover the lid of the plate to protect the coverslips from light. Incubating coverslips in this humid chamber will protect the coverslips from light in all steps. Use wet kimwipes to keep the humid chamber humid to prevent antibody evaporation.
- Add diluted LC3B antibody onto the coverslips. Incubate at room temperature for 1-4 h or at 4 °C overnight. If incubate overnight, make sure to add at least 50 μl to each coverslip and wet the kimwipes in the humid chamber in case of antibody evaporation.
- While incubating, prepare secondary antibodies by diluting fluorescently labeled anti-rabbit secondary antibody into AdDil-Tx.
- After incubation, wash LC3B antibody away by holding a transfer pipette filled with TBS-Tx in one hand and a pipe that is attached to a liquid bottle and a vacuum pump to remove washing solution. Let TBS-Tx flow through the coverslips to wash the antibody away. Each coverslip needs to be washed 3 times with around 2 ml of TBS-Tx each time.
- Add diluted secondary antibody onto coverslips and incubate at room temperature for around 1-2 h. After incubation, wash the coverslips in the same way as above.
- Take the mounting medium, ProLong® Gold Antifade Reagent with DAPI out of freezer around 10 minutes before the end of incubation to let it warm up to room temperature. Squeeze out a small drop (around 2-3 μl) of mounting medium onto the slide.
- Suck off the liquid on the coverslips as complete as possible and mound the coverslips onto the slide, on the top of ProLong® Gold Antifade Reagent with DAPI mounting medium. Remember to keep the cell side down and keep them with direct contact with mounting medium.
- The ProLong® Gold Antifade Reagent with DAPI mounting medium usually needs overnight to solidify and this step needs to be done at room temperature. Leave slides in a drawer or other space protected from light overnight to let it dry.
- When the slides are ready, take it to a fluorescent microscope to observe and take pictures (Figure 1).
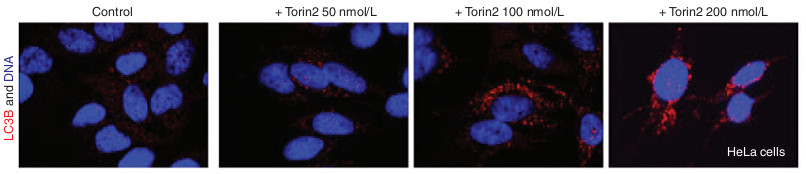
Figure 1. LC3B immunofluorescence shows that Torin2 induces autophagy in cells. Hela cells were treated with indicated concentrations of Torin2 for 3 days stained with antibody specific for LC3B and DAPI.
- Place one round coverslip per well in 24-well plate and add around 0.5 ml 70% ethanol for 1 min to sterilize the coverslip. Aspirate ethanol and let it dry for 5-10 min. Tissue cultured cells were plated on 24-well plate one day before experiment to let them attach to coverslips. [For cells to be plated on the coverslips, it depends on the time of drug treatment for your experiment so that the final cell confluency will be optimal for observation (80-90%)].
- LC3B western blot
- Tissue-culture cells are plated on 12-well plate (or 6-well plate) one day before experiment to let the cells attach to coverslips.
- On the day of experiment, 10 μg/ml of E64d/pepstatin A were added to cells one hour before to increase autophagy turnover. Different concentrations of small molecule compounds were then added to cells.
- After desired time of incubation, wash cells once with PBS. Then lyse cells in lysis buffer by directly overlay 100-200 μl lysis buffer (M-PER with protease inhibitor and phosphatase inhibitor) on the cells and incubate on ice (or 4 °C) for 10-20 min.
- At the end of incubation, collect cell lysate in a 1.5 ml tube and spin 1 minute at max speed and 4 °C to remove cell debris.
- Transfer the supernatant to a fresh tube, normalize, and add sample buffer. For drugs treated for a couple of hours, it’s usually not needed to normalize them since they normally don’t affect the cell numbers. However, for longer treatment (longer than a few hours), people should normalize the protein concentration across samples. Boil and load on a SDS-PAGE. Either a 15% SDS-PAGE or a 4-15% Mini-PROTEAN TGX Precast Gel id good for separating LC3B bands.
- Transfer gel into a PVDF membrane as the standard western blot protocol.
- Block the PVDF membrane in TBS-Tween (“TBS-T”) with 5% Non-fat dry milk for 1 h at room temperature on a shaker.
- Wash with TBS-T for 5 min, 3 times. Prepare LC3B antibody by diluting it at 1/1,000 into AbDil-Tw.
- Incubate membrane in diluted LC3B at room temperature for 2-3 h or at 4 °C overnight.
- Wash with TBS-T for 5 min, 3 times. Prepare HRP-conjugated secondary antibody by diluting it at 1/1,000 into AbDil-Tw.
- Incubate membrane in diluted secondary antibody at room temperature for 1 hour.
- Wash with TBS-T for 5 minutes, 3 times.
- Gently use paper towel to dry the membrane and place it face up and flat on a sheet of plastic wrap.
- Use prepared ECL to detect protein. Drain ECL and gently use paper towel and kimwipes to dry the membrane.
- Wrap up the membrane with plastic wrap.
- Either expose it to an X-ray film or use an imaging machine (Figure 2).

Figure 2. Western blot of LC3 shows that Torin2 induces apoptosis and autophagy in cells. Indicated cell lines were treated with increasing concentrations of AZD8055 or Torin2 for 3 days before the cells and analyzed by Western blot analysis using anti-LC3B and anti-tubulin antibodies.
- Tissue-culture cells are plated on 12-well plate (or 6-well plate) one day before experiment to let the cells attach to coverslips.
- Acridine orange assay
- Tissue cultured cells were plated on coverslips in 24-well plate one day before as introduced in LC3B immunofluorescence.
- One the day of experiment, cells are treated with different concentrations of small molecule compounds for desired amount of time before 1 μg/ml acridine orange was added for 15 min.
- Cells were washed once with PBS, fixed in PBS + 4% formaldehyde, washed with TBS-Tx before they are mounted in Prolong Gold with DAPI, which is the same as step A11 and A12.
- Pictures were taken by a fluorescent microscope (Figure 3, left panel).
- Total fluorescence of acridine orange in each frame was quantified using MetaMorph and divided by the total number of cells within the frame. Numbers are then normalized to DMSO-treated cells to show the acridine orange fold changes (Figure 3, right panel).
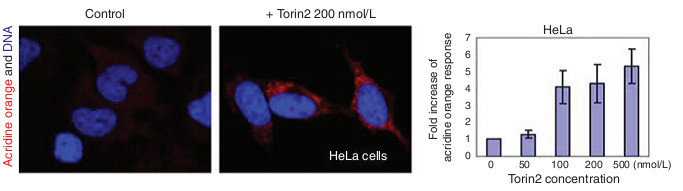
Figure 3. Acridine orange assay shows that Torin2 induces autophagy in cells. Hela cells were treated with different concent rations of Torin2 for 3 days and then stained for acridine orange and DAPI.
- Tissue cultured cells were plated on coverslips in 24-well plate one day before as introduced in LC3B immunofluorescence.
Recipes
- TBS
50 mM Tris-Cl
150 mM NaCl, pH 7.5
- TBS-Tween
TBS
0.1% Tween-20
- AbDil-Tw
TBS
0.1% Tween 20
2% BSA
0.1% NaN3
Filter, store at 4 °C
- TBS-Tx
TBS
0.1% Triton X-100
- AbDil-Tx
TBS
0.1% Tween 20
2% BSA
0.1% NaN3
Filter, store at 4 °C
- Running buffer
25 mM Tris base
190 mM glycine
0.1% SDS
- Transfer buffer
25 mM Tris base
190 mM glycine
20% methanol
Acknowledgments
This protocol was adapted from a previously published paper Qingsong et al. (2013). The implementation of this protocol was supported by “Thousand Talents Program” for Q. Liu and “Hundred Talents Program” for X. Zhang.
References
- Liu, Q., Xu, C., Kirubakaran, S., Zhang, X., Hur, W., Liu, Y., Kwiatkowski, N. P., Wang, J., Westover, K. D., Gao, P., Ercan, D., Niepel, M., Thoreen, C. C., Kang, S. A., Patricelli, M. P., Wang, Y., Tupper, T., Altabef, A., Kawamura, H., Held, K. D., Chou, D. M., Elledge, S. J., Janne, P. A., Wong, K. K., Sabatini, D. M. and Gray, N. S. (2013). Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res 73(8): 2574-2586.
- Qingsong, W., Yeming, G., Xuechun, L., Hongjuan, L. and Jing, W. (2013). The free radical scavenger, edaravone, ameliorates delayed neuropsychological sequelae after acute carbon monoxide poisoning in rabbits. Undersea Hyperb Med 40(3): 223-229.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, X. and Liu, Q. (2013). Autophagy Assays (LC3B immunofluorescence, LC3B western blot, acridine orange assay). Bio-protocol 3(24): e1012. DOI: 10.21769/BioProtoc.1012.
Category
Cell Biology > Cell imaging > Fluorescence
Cell Biology > Cell signaling > Autophagy
Biochemistry > Protein > Immunodetection > Western blot
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








