- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Adult Mouse Muscle Stem Cells
Published: Vol 3, Iss 24, Dec 20, 2013 DOI: 10.21769/BioProtoc.1003 Views: 10988
Reviewed by: Lin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1255 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Muscle stem cells are adult stem cells responsible for muscle development, growth and regeneration. Current knowledge suggests those cells are heterogeneous population shared their position between the sarcolemma and basal lamina of muscle fibers. This protocol describes the technique to dissociate and collect the stem cells from skeletal muscle of adult mice, and separate them from other cells found in muscle (e.g. fat, connective tissue). To purify and preserve those myofiber associated muscle stem cells, we use two steps of enzyme digestion followed by cell pre-plating procedures.
Materials and Reagents
- Collagenase type II (Sigma-Aldrich, catalog number: C6885 )
- Penicillin Streptomycin (Gibco®)
- HEPES
- Dulbecco’s Modified Eagle Medium (DMEM)
- Dispase II (STEMCELL Technologies)
- Bovine Growth Serum (Hyclone)
- Collagenase type II solution (see Recipes)
- Collagenase/Dispase solution (see Recipes)
- Growth medium (see Recipes)
Equipment
- Scissor
- 50 ml conical tube
- Microscope
- 100 mm cell culture dish
- 40 μm filter
- Fluorescence Activated Cell Sorter (FACS)
Procedure
- Remove skeletal muscle of interest (tibialis anterior, Soleus/gastrocnemlus, or quadriceps) from adult mice, and clean the hair tendon and fat with a fine scissor.
- Place muscle into a 50 ml conical tube and add two volume of collagenase digestion medium (1 g of muscle into 2 ml of digestion medium). Gently agitate the tube at 37 °C for 30 min to 1.5 h. The digestion time depends on if the muscle is from neonatal, adult or aged animals. During the digestion, regularly check the muscle under microscope until the muscle start to lossen up and single myofibers are separated.
- After digestion, stop the collagenase with 5 ml of growth medium and directly transfer the dissociated muscle into 100 mm cell culture dishes.
- Triturate up and down to disrupt the muscle with broken end glass pipets (the pore size of the broken end is around 5 mm in diameter), until all the muscle can easily pass through the pipette. The dissociation of muscle tissue to single myofibers can be confirmed under microscope.
- Tilt the plate to pool the tissue suspension, and transfer it into new tubes.
- If muscle is not completely dissociated, add 5 ml of growth medium and repeat steps 5 and 6.
- Spin down dissociated muscle fibers suspension at 100 x g for 3 min. Muscle fibers will be settled and formed a pellet.
- Discard supernatant (or spin at 500 x g for 5 min to collect mononuclear cells), and re-suspend the myofibers in 5 ml of growth medium.
- Repeat step 7 and 8 twice to remove all the mononuclear cells and small fiber fragments.
- Add 15 ml of collagenase II/Dispase II solution to 5 ml of myofiber suspension, digest the fibers at 37 °C for 1 h with gentle agitation. The long incubation time will make sure the myofibers are completely digested.
- Votex the digested fiber pellet for at least 1 min, then pass all the digested cells through 40 μm filter.
- Centrifuge digested cells at 500 x g for 5 min to pellet satellite cells. Wash the cell pellet with PBS twice to remove all the enzyme.
- Resuspend satellite cells in growth medium (~10ml for 1,000,000 cells), and preplate cells in uncoated plate for 20 min to remove fibroblast contamination.
- Collect unattached cells in growth medium for further application, such as cell culture and FACS (Chapman et al., 2013; Conboy et al., 2010).
- The quality and purity of isolated muscle stem cells could be analyzed with immunostaining of myogenic markers, such as Pax7 and Myf5 (Figure 1).
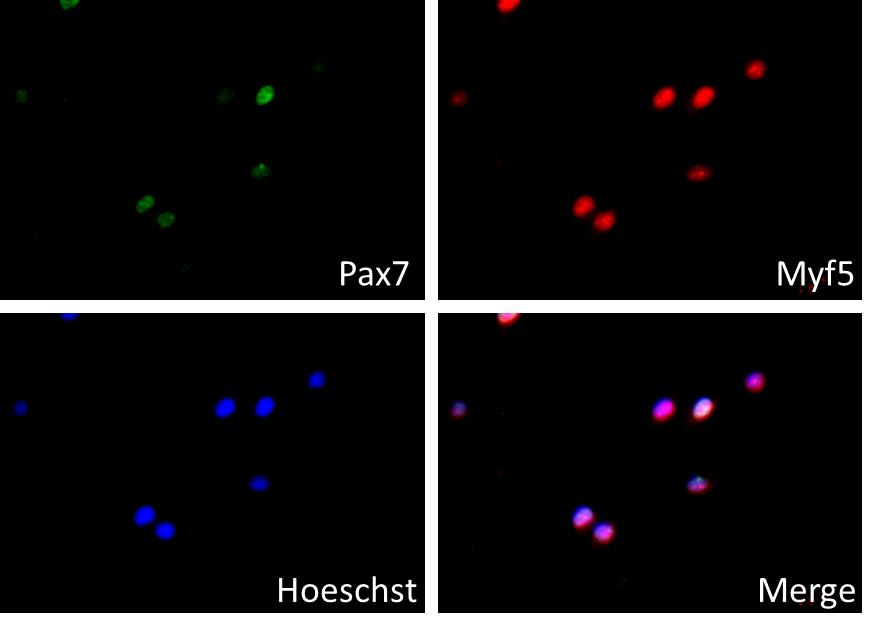
Figure 1. Immunostaining of isolated muscle stem cells
Recipes
- Collagenase digestion mediumCollagenase type II 250 U/ml
Penn/Strip: 1%
HEPES 10 mM (pH 6.4)
Solved in DMEM - Growth medium for muscle stem cells
20% bovine growth serum
1% Pen Strep
Solved in DMEM medium - Collagenase/Dispase solutionCollagenase type II (250 U/ml) 3.2 ml
Dipase II (10 U/ml) 4 ml
Growth medium 7.8 ml
Acknowledgments
This protocol is adapted from Chapman et al. (2013); Conboy et al. (2010) and Li et al. (2009).
References
- Chapman, M. R., Balakrishnan, K. R., Li, J., Conboy, M. J., Huang, H., Mohanty, S. K., Jabart, E., Hack, J., Conboy, I. M. and Sohn, L. L. (2013). Sorting single satellite cells from individual myofibers reveals heterogeneity in cell-surface markers and myogenic capacity. Integr Biol (Camb) 5(4): 692-702.
- Conboy, M. J. and Conboy, I. M. (2010). Preparation of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol 621: 149-163.
- Conboy, M. J., Cerletti, M., Wajers, A. J., Conboy, I. M. (2010). Immuno-analysis and FACS sorting of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol 621: 165-173.
- Li, J., Reed, S. A. and Johnson, S. E. (2009). Hepatocyte growth factor (HGF) signals through SHP2 to regulate primary mouse myoblast proliferation. Exp Cell Res 315(13): 2284-2292.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Li, J. (2013). Preparation of Adult Mouse Muscle Stem Cells. Bio-protocol 3(24): e1003. DOI: 10.21769/BioProtoc.1003.
Category
Stem Cell > Adult stem cell > Muscle stem cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









