- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
ImmunoFISH for Mice and Baboons Frozen Sections
Published: Vol 3, Iss 24, Dec 20, 2013 DOI: 10.21769/BioProtoc.1000 Views: 9566
Reviewed by: Lin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measuring the Endocytic Recycling of Amyloid Precursor Protein (APP) in Neuro2a Cells
Florent Ubelmann [...] Claudia Guimas Almeida
Dec 5, 2017 8138 Views

Non-separate Mouse Sclerochoroid/RPE/Retina Staining and Whole Mount for the Integral Observation of Subretinal Layer
Sung-Jin Lee and Soo-Young Kim
Jan 5, 2021 4457 Views

Time-Lapse Into Immunofluorescence Imaging Using a Gridded Dish
Nick Lang [...] Andrew D. Stephens
Feb 20, 2026 53 Views
Abstract
This protocol is optimized for immunoFISH staining of OCT section of mouse tissues. It combines immunofluorescence for DNA damage response factors (e.g. 53BP1) (Le et al., 2010) and FISH against telomeric DNA.
Keywords: ImmunoFISHMaterials and Reagents
- Tissue
- OCT
- 4% formaldehyde
- PBS
- Triton
- Goat serum
- BSA
- Primary antibody: 53BP1 #NB 100-304 rabbit from Novus
- Second antibody: goat anti-rabbit Alexa Fluor® 488 Dye
- Triton
- Glycine
- Mowiol 4-88 reagent (Calbiochem®)
- Formamide
- Tris HCl, pH 7.4
- Telomeric PNA probe (TelC-Cy3 from PANAGENE, catalog number: F1002-5 )
- Tween-20
- DAPI
- Hybridization mixture (see Recipes)
- Blocking reagent (Roche Diagnostics, catalog number: 11096176001 ) (see Recipes)
- Wash solution I (see Recipes)
- Wash solution II (see Recipes)
Equipment
- Glass slide
- Metal thermoblock
- Humidified chamber
Procedure
- Frozen tissue placed in OCT without fixation.
- When needed, slice to the desired thickness (8-10 micron), dry the slides few minutes (often the time to prepare the other slides) and freeze again at -80 °C.
- The day of the staining, thaw the slides and fix for 20 min in 4% formaldehyde.
- Wash slides with PBS for 3 x 5 min at RT.
- Permeabilize slides with 0.5% Triton in PBS for 5 min at RT.
- Wash 2x with PBS 5 min at RT.
- Block in 5% Goat serum diluted in PBS + 1% BSA for 60 min.
- Incubate at 4 °C: 53BP1 #NB 100-304 (rabbit from Novus) 1:100 in PBS, 2.5% goat serum, 1% BSA. Use 60-80 μl for each slide.
- Wash once quickly and 3 x 10 min with PBS at RT.
- Secondary: goat anti-rabbit (Alexa 488) (1/100) in PBS + 1% BSA for 60 min at RT.
- Wash once quickly and 3 x 10 min with PBS RT.
- Re-fix tissue with PFA 4% + Triton 0.1%, 10 min RT.
- Incubate with glycine 10 mM in H2O, 30 min, RT.
- Wash with 1x PBS, 3 times, 5 min.
- Prepare the hybridization mixture and put 30-50 μl directly on the sample.
- Put a glass slide carefully on the drop without making bubbles.
- Put the slide directly on a metal thermoblock at 80 °C, 5 min.
- Hybridize in a humidified chamber, 2 h, RT.
- Remove glass from the slide.
- Wash with Wash solution I, twice, 15 min.
- Wash with Wash solution II, 3 times, 5 min.
- Incubate with DAPI, 2 min, RT.
- Wash briefly with 1x PBS.
- Mount with mowiol.
- Store the slides at 4 °C for short time storage (2 weeks) or at -20 °C. It is recommended to analyze the fluorescence as soon as possible to avoid fluorophore fading.
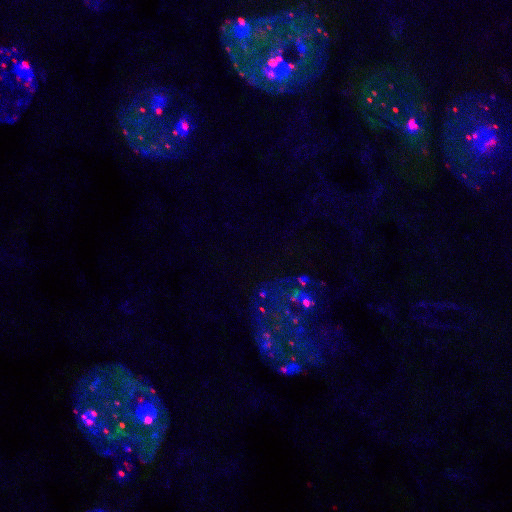
Figure 1. A representative figure of ImmunoFISH stained mouse hippocampus tissue. DAPI is in blue, 53BP1is in green and telomeric PNA probe is in red.
Recipes
- Hybridization mixture (always prepare fresh)
Formamide
70%
Blocking reagent
1x
Tris HCl pH 7.4
10 mM
Telomeric PNA probe
0.5 μM
H2O
to volume
- 10x Blocking reagent
Prepare small aliquots and store them at -20 °C.
- Wash Solution I (250 ml) (always prepare fresh)
Formamide
175 ml
BSA 10%
2.5 ml
Tris HCl 1 M pH 7.4
2.5 ml
H2O
to volume
- Wash Solution II (350 ml) (always prepare fresh)
Tris HCl 1 M pH 7.4
35 ml
NaCl 5 M
10.5 ml
Tween-20 10%
2.5 ml
H2O
to volume
Acknowledgments
The immunofluorescence part of the protocol is adapted from Le et al. (2010). The F.d’A.d.F. laboratory is supported by FIRC (Fondazione Italiana per la Ricerca sul Cancro), AIRC (Associazione Italiana per la Ricerca sul Cancro), European Union (GENINCA, contract number 202230), HFSP (Human Frontier Science Program), AICR (Association for International Cancer Research), EMBO Young Investigator Program and Telethon.
References
- Fumagalli, M., Rossiello, F., Clerici, M., Barozzi, S., Cittaro, D., Kaplunov, J. M., Bucci, G., Dobreva, M., Matti, V. and Beausejour, C. M. (2012). Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 14(4): 355-365.
- Le, O. N., Rodier, F., Fontaine, F., Coppe, J. P., Campisi, J., DeGregori, J., Laverdière, C., Kokta, V., Haddad, E. and Beauséjour, C. M. (2010). Ionizing radiation‐induced long‐term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell 9(3): 398-409.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Rossiello, F., Fumagalli, M. and di Fagagna, F. D. (2013). ImmunoFISH for Mice and Baboons Frozen Sections. Bio-protocol 3(24): e1000. DOI: 10.21769/BioProtoc.1000.
Category
Cell Biology > Cell imaging > Fluorescence
Cell Biology > Tissue analysis > Tissue isolation
Biochemistry > Protein > Immunodetection > Immunostaining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link