- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Serial Transfer of Human Hematopoietic and Hepatic Stem/progenitor Cells
Published: Vol 3, Iss 23, Dec 5, 2013 DOI: 10.21769/BioProtoc.992 Views: 15110
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Functional Analysis of Connexin Channels in Cultured Cells by Neurobiotin Injection and Visualization
Philipp Wörsdörfer and Klaus Willecke
Jun 5, 2017 8870 Views

3D Organoid Formation from the Murine Salivary Gland Cell Line SIMS
Harleen K. Athwal and Isabelle M. A. Lombaert
Oct 5, 2019 6247 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 242 Views
Abstract
A range of assays have been developed to determine the stemness or stem cell activity of human stem cells. The key assays of stem cells are functional: they must show self-renewal and the ability to generate the appropriate tissue. The best assays available to study this property in putative human stem cells involve xeno-transplantation into immune-deficient mice. Demonstration of both long-term (2-3 months) multi-lineage reconstitution of human blood or liver in a murine host and the ability of the putative stem cells to mediate reconstitution of a secondary host upon re-isolation from the primary mouse are generally accepted as the gold standard for demonstrating the presence of human hematopoietic and hepatic stem cells. Here, we describe a method of reconstituting NOD-scid IL-2Rγ-/-(NSG) mice with CD34+ stem cells from human fetal liver and repurification of CD34+ cells for serial transplantation.
Keywords: Hematopoietic progenitorMaterials and Reagents
- NOD.Cg-Prkdcscid //2rgtm1Wjl/SzJ (The Jackson Laboratory, stock number: 00 5557 )
- CD34+ fetal liver cells
- StemSpanTM SFEM (STEMCELL Technologies, catalog number: 0 9650 )
- ACK Lysing Buffer (Life Technologies, catalog number: A1049201 )
- Liver perfusion medium (Life Technologies, catalog number: 17701-038 )
- Liver digestion medium (Life Technologies, catalog number: 17703-034 )
- RoboSepTM Buffer (STEMCELL Technologies, catalog number: 20104 )
- Trypan blue solution (Sigma-Aldrich, catalog number: T8154 )
- EasySepTM Human Cord Blood CD34 Positive Selection Kit (STEMCELL Technologies, catalog number: 18096 )
- DMEM
Equipment
- Biosafety cabinet
- Petri dishes (100-mm2)
- 137 Cs gamma irradiator
- Insulin syringe (29 G 1 cc) (BD Biosciences, catalog number: 320310 )
- Syringe (5 ml) (BD, catalog number: 309646 )
- Heating pad or warming lamp
- Butterfly needle (BD, catalog number: 368659 )
- Cell strainer (100 μm) (BD Biosciences, catalog number: 352360 )
- Falcon tube(15 ml)
Procedure
- Engraftment of primary recipient mouse
- CD34+ fetal liver cells are purified based on the protocol “Isolation of CD34+ Cells from Human Fetal Liver and Cord Blood” (Chen and Chen, 2013)
- Monitor breeder pairs for the birth of new litters.
Note: Engraftment procedures should be performed on newborn pups 24 to 48 h post-natal. - Prepare CD34+ fetal liver cells suspend in StemSpan at 2.5 x 105 cells/50 μl/pup.
Note: Freshly prepared or previously frozen preparations may be used. - Place 24- to 48-h post-natal pups from a single litter into a 100 mm2 petri dish along with a small amount of bedding material from the breeder cage.
- The petri dish is put into a 137 Cs gamma irradiator. Irradiate pups with 1 Gy whole body irradiation.
- The petri dish is then brought back into biosafety cabinet. A second sterile petri dish is prepared with cotton nestlet from parent cage.
- One pup is taken at a time from the irradiated dish and held firmly, yet with great care, by thumb and index finger of one hand. Tilt the pup back so that abdomen is exposed. The liver will be visible on the right flank of the pup (Figure 1). Disinfect area with alcohol pad.
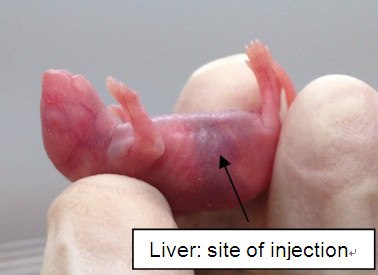
Figure 1. Liver: site of injection - The other hand holds a 29 G 1 cc insulin syringe loaded with 50 μl of fetal liver cells.
- The needle (perpendicular to body) will be inserted straight in, with bevel facing upwards, approximately 3 mm into the pup.
- The cells are then carefully and slowly injected into the pup's liver.
- Once injected, the needle is removed and gentle pressure is applied to the area. The injected pup is then placed in the second petri-dish.
- Steps A7-11 are repeated until all the pups have been injected.
- The pups are carefully placed back into their parents’ cage and covered with the cotton nestlet so they will smell familiar to parents.
- The pups will be monitor everyday for seven days. Any pups exbihiting severe weight loss, dehyration, dyspnea should be euthanized immediately. From experience, the pups are mostly unaffected by the injection.
- CD34+ fetal liver cells are purified based on the protocol “Isolation of CD34+ Cells from Human Fetal Liver and Cord Blood” (Chen and Chen, 2013)
- Cell repurification and reconstitution of secondary recipient mouse
- 8 to 10 weeks later, the primary mice are used for repurification of human hematopoietic stem cells and hepatic progenitor cells respectively.
For repurification of hematopoietic stem cells from femurs - Femurs are harvested and Remove as much muscle as possible around the femur bone.
- Attach a 27 G needle to a 5 ml syringe filled with PBS.
- Place the needle into the bone marrow (red middle of the bone), and flush out cells onto a 100 μm cell strainer in a 5 cm petri dish.
- Mesh cells and transfer the pass-through to a 15 ml Falcon tube.
- Pellet cells at 400 x g for 5 min.
- Lyze red blood cells with ACK lysis buffer.
- Re-purify CD34+ cells by magnetic selection with EasySepTM Human Cord Blood CD34 Positive Selection Kit.
For repurification of hepatic progenitor cells from livers - Cannulate portal vein (Figure 2) with a 27 G buffer fly needle.
- Make incision in inferior vena cava (Figure 2).
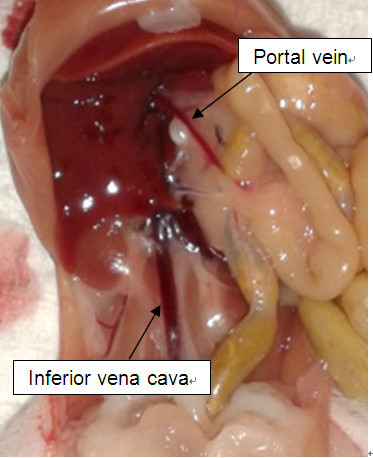
Figure 2. Portal vein and inferior vena cava - Mouse livers were first perfused with pre-warmed liver perfusion medium at 0.7 ml/min for 10 min, then with pre-warmed liver digestion medium for 10 min.
- Carefully transfer the digested liver to a petri dish and dis-associate liver cells into single cell suspensions with curved forceps.
- The cell suspensions were washed with ice-cold DMEM at 50 x g for 5 min.
- Assay cell viability by Trypan Blue dye.
- CD34+ cells were re-purified from the cell suspensions with EasySepTM Human Cord Blood CD34 Positive Selection Kit.
- 8 to 10 weeks later, the primary mice are used for repurification of human hematopoietic stem cells and hepatic progenitor cells respectively.
- Reconstitution of secondary recipients
- CD34+ cells of desired numbers were injected into sublethally irradiated newborn NSG pups.
- After 8 to 10 weeks, samples e.g. blood and livers are harvested for analysis.
- CD34+ cells of desired numbers were injected into sublethally irradiated newborn NSG pups.
Acknowledgments
This protocol was developed and adapted from the previous publication Chen et al (2013a) and (2013b).
References
- Chen, Q., Khoury, M., Limmon, G., Choolani, M., Chan, J. K. and Chen, J. (2013a). Human fetal hepatic progenitor cells are distinct from, but closely related to, hematopoietic stem/progenitor cells. Stem Cells 31(6): 1160-1169.
- Chen, Q. and Chen, J. (2013b). Isolation of CD34+ cells from human fetal lLiver and cord blood. Bio-protocol 3(23): e991.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chen, Q. and Chen, J. (2013). Serial Transfer of Human Hematopoietic and Hepatic Stem/progenitor Cells. Bio-protocol 3(23): e992. DOI: 10.21769/BioProtoc.992.
Category
Stem Cell > Adult stem cell > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










