- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Adherent-invasive Escherichia coli Biofilm Formation Assays
Published: Vol 3, Iss 23, Dec 5, 2013 DOI: 10.21769/BioProtoc.982 Views: 14253
Reviewed by: Fanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Inexpensive Imaging Platform to Record and Quantitate Bacterial Swarming
Weijie Chen [...] Jay X. Tang
Sep 20, 2021 3651 Views

Purification of the Bacterial Amyloid “Curli” from Salmonella enterica Serovar Typhimurium and Detection of Curli from Infected Host Tissues
Murugesan Sivaranjani [...] Aaron P. White
May 20, 2022 3241 Views

A Guideline for Assessment and Characterization of Bacterial Biofilm Formation in the Presence of Inhibitory Compounds
Bassam A. Elgamoudi and Victoria Korolik
Nov 5, 2023 3151 Views
Abstract
Patients with Crohn’s disease are abnormally colonized by adherent-invasive Escherichia coli (AIEC) bacteria (Chassaing and Darfeuille-Michaud, 2011). These bacteria are able to adhere to and invade intestinal epithelial cells (IEC), to replicate within macrophages, and were recently described to be able to form biofilms (Martinez-Medina et al., 2009; Chassaing and Darfeuille-Michaud, 2013). The reference strain of adherent-invasive E. coli is the strain LF82, associated with ileal Crohn’s disease (Darfeuille-Michaud et al., 1998).
This protocol described basic steps of a biofilm formation assay on I) non-cell-treated polystyrene microtiter plates and on II) paraformaldehyde-fixed I-407 IEC monolayers.
Materials and Reagents
- Adherent-invasive E. coli reference strain LF82 (Darfeuille-Michaud et al., 1998)
- Intestine-407 (I-407) cells (ATCC, catalog number: CCL-6 )
- Luria Broth (BD DifcoTM, catalog number: 244620 )
- Glucose (Sigma-Aldrich, catalog number: G8270 )
- M63 medium (United States Biological, catalog number: M1015 )
- MgSO4 (Sigma-Aldrich, catalog number: 208094 )
- Phosphate-Buffered Saline (PBS) ( Mediatech, Cellgro®, catalog number: 21-040 )
- Crystal Violet (Sigma-Aldrich, catalog number: C6158 )
- 200 proof absolute Ethanol (Sigma-Aldrich)
- Fetal bovine serum (FBS) (Mediatech, Cellgro®, catalog number: 35-010-CV )
- Nonessential amino acids (Mediatech, Cellgro®, catalog number: 25-025-CI )
- L-glutamine (Mediatech, Cellgro®, catalog number: 25-005 )
- Penicillin/Streptomycin/Amphotericin B solution (Mediatech, Cellgro®, catalog number: 30-004-CI )
- Vitamin mix (Mediatech, Cellgro®, catalog number: 25-020-CI )
- Formaldehyde solution (Sigma-Aldrich, catalog number: F8775 )
- Phalloidin-tetramethyl rhodamine isocyanate (Sigma-Aldrich, catalog number: P1951 )
- Paraformaldehyde
- Hoechst 33258 (Sigma-Aldrich, catalog number: B1155 )
- Vectashield (Vector Labs, catalog number: H-1000 )
- Minimum Essential Medium (MEM) (Mediatech, Cellgro®, catalog number: 10-022-CV ) (see Recipes)
Equipment
- Coverslips (Electron Microscopy Sciences)
- Sterile tubes
- 24-well tissue culture plates
- Non-cell-treated polystyrene 96-well microtiter plates (Falcon®, catalog number: 62406-117 )
- 24-well polystyrene plate, tissue-culture treated (Falcon®, catalog number: 62406-159 )
- Microplate shaker (LABREPCO, model: BT1500 )
- Microplate spectrophotometer
- Confocal microscope
- 30 °C incubator
- 37 °C/5% CO2 incubator
Software
- Computer program COMSTAT1
Procedure
- Biofilm formation assay on non-cell-treated polystyrene microtiter plates
- Bacterial strains were grown overnight in sterile tubes containing 2 ml of Luria-Bertani broth with 5 g.L-1 glucose at 35.5 °C without agitation (Martinez-Medina et al., 2009; Chassaing and Darfeuille-Michaud, 2013).
- The following day, bacterial suspension was diluted 1/100 in M63 minimal medium supplemented with 1 mM of MgSO4 and 8 g.L-1 glucose.
- Aliquots of 130 μl were then placed in wells of non-cell-treated polystyrene 96-well microtiter plates and incubated 24 h at 30 °C without shaking. One well designed as blank received only M63 minimal medium supplemented with 1 mM of MgSO4 and 8 g.L-1 glucose without bacteria.
- After incubation, microplates were agitated for 5 min using a microplate shaker, and bacterial growths were then estimated by OD620nm reading using a microplate spectrophotometer.
- The liquid was remove by decantation, and the wells were washed once using 200 μl sterile PBS without agitation.
- In order to dry them, plates were left open for at least 20 min at room temperature.
- Adherent bacteria forming biofilm were stained with 130 μl of 1% crystal violet solubilized in ethanol for 5 min at room temperature without agitation.
- Wells were then washed five times using 200 μl sterile PBS without agitation.
- In order to dry them, plates were left open for at least 1 h at room temperature.
- 130 μl of absolute ethanol was added, and plates were incubated 5 min at room temperature without agitation.
- High-speed agitation was performed using a microplate shaker for 10 min (1000 rpm).
- Biofilm formation was estimated by OD570nm reading using a microplate spectrophotometer.
13.Specific biofilm formation index could be determined by using the ration OD570nm/OD620nm that allows standardization of biofilm formation according to bacterial growth in M63 minimal medium. According to previous publication, specific biofilm formation index of wild type AIEC strain LF82 should be between 2 and 4 (Martinez-Medina et al., 2009; Chassaing and Darfeuille-Michaud, 2013).
- Bacterial strains were grown overnight in sterile tubes containing 2 ml of Luria-Bertani broth with 5 g.L-1 glucose at 35.5 °C without agitation (Martinez-Medina et al., 2009; Chassaing and Darfeuille-Michaud, 2013).
- Biofilm formation assay on paraformaldehyde-fixed I-407 IEC monolayers
Intestine-407 (I-407) cells were derived from human intestinal embryonic jejunum and ileum tissues. They were maintained in an atmosphere containing 5% CO2 at 37 °C in Minimum Essential Medium supplemented with 10% (vol/vol) fetal bovine serum; 1% of nonessential amino acids; 1% of L-glutamine; 1% of Penicillin/Streptomycin/Amphotericin B solution and 1% of vitamin mix.
- Monolayers were seeded on coverslip in 24-well tissue culture plates with 4 x 105 cells per well and incubated for 20 h at 37 °C with 5% CO2.
- Monolayers were then washed once with 1ml PBS, fixed for 15 min in 4% formaldehyde (stock solution diluted in sterile PBS) at room temperature without agitation, and then washed 4 times with PBS.
- Bacterial strains expressing green fluorescent protein (GFP) (Valdivia et al., 1996) were prepared as previously described above, from step A1 to A2, in M63 minimal medium supplemented with 1 mM of MgSO4 and 8 g.L-1 glucose.
- 1 ml of this bacterial suspension was applied on the surface of fixed I-407 cell monolayers, and incubated overnight at 30 °C without shaking.
- The liquid was removed by decantation, and the wells were washed 3 times using 1 ml of sterile PBS without incubation.
- I-407/biofilm complex was then fixed for 15 min using 4% formaldehyde.
- Phalloidin-tetramethyl rhodamine isocyanate was used to visualize actin and Hoechst 33258 was used to visualize nuclei. For this purpose, Phalloidin-tetramethyl rhodamine isocyanate was diluted in sterile PBS at a final concentration of 1μg/ml and Hoechst 33258 was added at a final concentration of 1μg/ml. This solution was added to the wells and incubated for 20 min at room temperature. After incubation, the wells were washed 5 times using 1 ml of sterile PBS without incubation.
- Coverslips containing the fixed and stained I-407/biofilm complex were then removed from the 24-well plate and mounted on slide using Vectashield.
- The slides were examined with a confocal microscope. An example of biofilm formation on paraformaldehyde-fixed I-407 IEC monolayers is presented Figure 1.
- Images from biofilm formation assay at the surface of intestinal epithelial cells I-407 monolayers could be analyzed for thickness and/or roughness with the computer program COMSTAT1 (Haydorn et al., 2000).
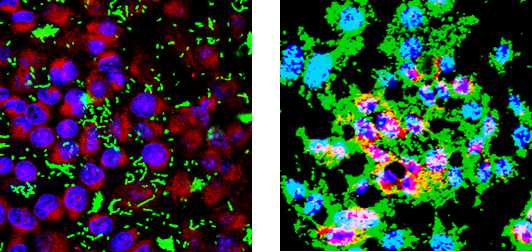
Figure 1. Confocal analysis of biofilm formation of a poor biofilm producer (Non pathogenic Escherichia coli K12 strain C600 (A) and a strong biofilm producer (Adherent-Invasive Escherichia coli strain LF82 (B) at the surface of a PFA-fixed monolayer of I-407 intestinal epithelial cells. Bacteria express GFP (green), actin is stained in red using phalloidin-TRITC, and DNA is stained in blue using Hoechst. Bars, 50 m.
- Monolayers were seeded on coverslip in 24-well tissue culture plates with 4 x 105 cells per well and incubated for 20 h at 37 °C with 5% CO2.
Notes
- Due to high variation observed with both of these biofilm formation assays, they need to be performed at least in triplicate, including controls.
Recipes
- Minimum Essential Medium supplemented with
10% (vol/vol) fetal bovine serum
1% of nonessential amino acids
1% of L-glutamine
1% of Penicillin/Streptomycin/Amphotericin B solution
1% of vitamin mix
Acknowledgments
This study was supported by the Ministère de la Recherche et de la Technologie, the Institut National de la Santé et de la Recherche Médicale and the Université d’Auvergne (UMR Inserm 1071), the Institut National de la Recherche Agronomique (USC INRA 2018) and by grants from the Association F. Aupetit (AFA). We thank the CICS platform for confocal microscopy. This protocol is adapted from previously published papers (Danese et al., 2000; Naves et al., 2008; Martinez-Medina et al., 2009; Chassaing and Darfeuille-Michaud, 2013). This work is dedicated to Arlette Darfeuille-Michaud.
References
- Chassaing, B. and Darfeuille-Michaud, A. (2011). The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140(6): 1720-28.
- Chassaing, B. and Darfeuille-Michaud, A. (2013). The sigmaE pathway is involved in biofilm formation by Crohn's disease-associated adherent-invasive Escherichia coli. J Bacteriol 195(1): 76-84.
- Danese, P. N., Pratt, L. A., Dove, S. L. and Kolter, R. (2000). The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol Microbiol 37(2): 424-432.
- Darfeuille-Michaud, A., Neut, C., Barnich, N., Lederman, E., Di Martino, P., Desreumaux, P., Gambiez, L., Joly, B., Cortot, A. and Colombel, J. F. (1998). Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 115(6): 1405-1413.
- Martinez-Medina, M., Naves, P., Blanco, J., Aldeguer, X., Blanco, J. E., Blanco, M., Ponte, C., Soriano, F., Darfeuille-Michaud, A. and Garcia-Gil, L. J. (2009). Biofilm formation as a novel phenotypic feature of adherent-invasive Escherichia coli (AIEC). BMC Microbiol 9: 202.
- Heydorn, A., Nielsen, A. T., Hentzer, M., Sternberg, C., Givskov, M., Ersboll, B. K. and Molin, S. (2000). Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146 ( Pt 10): 2395-2407.
- Naves, P., del Prado, G., Huelves, L., Gracia, M., Ruiz, V., Blanco, J., Dahbi, G., Blanco, M., Ponte Mdel, C. and Soriano, F. (2008). Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb Pathog 45(2): 86-91.
- Valdivia, R. H., Hromockyj, A. E., Monack, D., Ramakrishnan, L. and Falkow, S. (1996). Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene 173(1 Spec No): 47-52.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chassaing, B. and Darfeuille-Michaud, A. (2013). Adherent-invasive Escherichia coli Biofilm Formation Assays. Bio-protocol 3(23): e982. DOI: 10.21769/BioProtoc.982.
Category
Microbiology > Microbial biofilm > Biofilm culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link