- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Mouse Tumor-Infiltrating Leukocytes by Percoll Gradient Centrifugation
Published: Vol 3, Iss 17, Sep 5, 2013 DOI: 10.21769/BioProtoc.892 Views: 44900
Reviewed by: Lin FangFanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Murine Alveolar Type II Epithelial Cells
Fan Sun [...] Zhaoxia Qu
May 20, 2017 13116 Views

Soft Agar Colony Formation Assay as a Hallmark of Carcinogenesis
Feng Du [...] Daiming Fan
Jun 20, 2017 30592 Views

A Fast and Reliable Method to Generate Pure, Single Cell-derived Clones of Mammalian Cells
Zhe Han [...] Varun Kumar
Aug 20, 2022 4105 Views
Abstract
A hallmark of cancer-associated inflammation is the infiltration of leukocytes into tumors, which is believed to be recruited by chemokines. Some infiltrating leukocytes such as macrophages often promote tumor growth by producing growth-inducing and angiogenic factors. Here, we described a method of isolating tumor-infiltrating leukocytes with Percoll density gradient, because Percoll possesses a low viscosity, a low osmolarity and no toxicity to cells. Different leukocyte populations are isolated based on their density and collected at the interface between 40% and 80% discontinuous Percoll gradient.
Materials and Reagents
- Mice with tumors
- Collagenase, type IV (Sigma-Aldrich, catalog number: C5138 )
- DNase, type IV (Sigma-Aldrich, catalog number: D5025 )
- Fungizone Antimycotic (Life Technologies, InvitrogenTM, catalog number: 15290-026 )
- Hank's Balanced Salt Solution (HBSS), without Calcium Chloride or Magnesium Chloride (Lonza, catalog number: 10-547F )
- Hyaluronidase, Type V (Sigma-Aldrich, catalog number: H6254 )
- Bovine Serum Albumin (BSA) (Sigma-Aldrich, catalog number: A4503 )
- 0.5 M EDTA (Quality Biological Inc, catalog number: qb351-027-721 )
- Percoll (GE Healthcare, catalog number: 17-0891-01 )
- Dulbecco’s Modified Eagle’s Medium High glucose with stable L-glutamine (DMEM) (Lonza, catalog number: 12-614F )
- Fetal Bovine Serum (FBS) (Thermo Fisher Scientific, catalog number: SH30070-03 )
- 10x PBS (Life Technologies, Gibco®, catalog number: 70011 )
- ACK lysing buffer (Lonza, catalog number: 10-548E )
- 10x Triple Enzyme Stock Solution (see Recipes)
- Wash buffer (see Recipes)
- 40% Percoll (see Recipes)
- 80% Percoll (see Recipes)
Equipment
- 25 ml canted Neck and Blue Plug Seal Cap, non-vented tissue culture flasks (BD Biosciences, Falcon®, catalog number: 353018 )
- Shaker (Lab-Line, model: 1314 )
- Centrifuge
- 100 mm Petri dish
Procedure
- Dissect Lewis Lung Cancer (LLC) tumors (tumor volume ~1,000 mm3/tumor, tumor weight: 0.5~0.8 g/tumor) grown on mice.
- Place one tumor into a 100 mm Petri dish and add 5-10 ml HBSS at room temperature (RT).
- Quickly mince tumors with scalpels into fragments small enough to be aspirated into a 5 ml pipette without getting stuck at RT.
- Transfer tumor tissue suspension into 50 ml non-vented tissue culture flasks.
- Rinse Petri dish with up to 40 ml HBSS and transfer the suspension into the flask. Total volume in the flask is 45 ml.
- Add 5 ml 10x Triple Enzyme Mix to the flask, and shake the flask at 80 rpm at RT for 1-3 h on a shaker.
- Repeatedly pipet the tumor cell suspension to further dissociate cells, centrifuge the cell suspension at 50 x g at RT for 10 min and collect the supernatant. Discard the bigger pellets in the bottom of the tube and centrifuge the supernatant at 200 x g for 5 min.
- Wash cell pellets with 10 ml wash buffer at 200 x g for 5 min once.
- Suspend the cell pellet with 2 ml ACK lysing buffer for 1 min to deplete red blood cells (observe the cell suspension under a microscope to determine whether red blood cells are depleted completely. If there are still too many red blood cells, add more ACK lysing buffer until there are very few red blood cells).
- Centrifuge at 200 x g and remove the supernatant.
- Re-suspend cells in 15 ml 40% Percoll and add into a 50 ml tube.
- Lay slowly 15 ml 80% Percoll with a syringe and a long needle (#16 gauge) to the bottom of the above 50 ml tube.
- Centrifuge at 325 x g at RT for 23 min (ascending rate: 5; descending rate: 5).
- Collect the cells at the interface between 40% and 80% Percoll, which should mostly be leukocytes and directly pass the cell suspension through a 40 μm nylon strainer. Wash the strainer with wash buffer and centrifuge the cell suspension at 425 x g at RT for 10 min.
Note: After centrifugation, approximately 1.5-2.0 x 105 leukocytes could be obtained from a total of 1 x 106 cells from tumor depending on the tumor type. By discontinuous Percoll isolation, tumor cells will be in the bottom layer after centrifugation (Figure 1).
- Re-suspend the cells in desired buffer for further experiments, such as phenotyping with flow cytometry and measurement of functions.
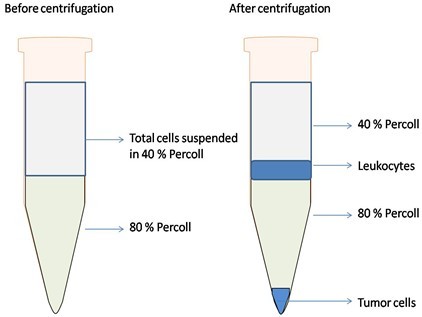
Figure 1. Schematic illustration of the isolation of tumor infiltrating-leukocytes by discontinuous Percoll gradient. Left tube shows that before centrifugation, 80% Percoll is laid under the total cells suspended in 40% Percoll. Right tube shows that after centrifugation, leukocytes are located at the interface between 40% and 80% Percoll, tumor cells are in the bottom of the tube.
Recipes
- 10x Triple Enzyme stock solution (100 ml)
Mix 1 g Collagenase IV, 100 mg Hyaluronidase and 20,000 Units DNase IV into 80 ml HBSS
Add HBSS to 100 ml
Filter (0.22 μm) sterilize the stock solution
Store 5 ml aliquots at -20 °C
Thaw at RT (NOT 37 °C) before use.
- Wash buffer (1,000 ml)
Mix 1 g BSA and 2 ml 0.5 M EDTA with 800 ml HBSS
Add HBSS to 1,000 ml
- 40% Percoll, 5 ml
Mix 0.6 ml 10x PBS, 5.4 ml Percoll and 9 ml DMEM
- 80% Percoll, 15 ml
Mix 1.2 ml 10x PBS, 10.8 ml Percoll and 3 ml DMEM
Acknowledgments
This protocol is adapted from Liu et al. (2013).
References
- Liu, Y., Chen, K., Wang, C., Gong, W., Yoshimura, T., Liu, M. and Wang, J. M. (2013). Cell surface receptor FPR2 promotes antitumor host defense by limiting M2 polarization of macrophages. Cancer Res 73(2): 550-560.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Liu, Y., Chen, K., Wang, C., Gong, W., Yoshimura, T., Wang, J. M. and Liu, M. (2013). Isolation of Mouse Tumor-Infiltrating Leukocytes by Percoll Gradient Centrifugation. Bio-protocol 3(17): e892. DOI: 10.21769/BioProtoc.892.
Category
Cancer Biology > General technique > Cell biology assays > Cell isolation and culture
Cancer Biology > Tumor immunology > Biochemical assays > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










