- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
HIV-1 Virus-like Particle Budding Assay
Published: Vol 3, Iss 14, Jul 20, 2013 DOI: 10.21769/BioProtoc.830 Views: 11135
Reviewed by: Fanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Coupling of HIV-1 gp120-derived Core Protein to Paramagnetic Beads and Adsorption Assays
Jidnyasa Ingale and Richard T Wyatt
Oct 5, 2015 7341 Views

Construction of a Highly Diverse mRNA Library for in vitro Selection of Monobodies
Taishi Kondo [...] Hiroshi Murakami
Aug 20, 2021 3887 Views
Abstract
Viral replication culminates with the egress of the mature virion from the host cell. This step of the viral life cycle has recently garnered increased attention with the discovery of the cellular restriction factor, Tetherin, which tethers budded virions to the surface of infected cells and inhibits viral spread. The importance of this block in viral infections has been suggested by the discovery of viral antagonists, such as HIV-1 Vpu, which counteract Tetherin. This protocol describes a system to study HIV-1 budding under BSL-2 safety conditions. It takes advantage of the ability of many viral matrix/capsid proteins to generate non-infectious virus-like particles (VLPs) with the expression of a single viral protein (i.e. HIV-1 p24 Gag). This protocol was recently used to characterize the effect of Tetherin isoforms on VLP release in the presence of HIV-1 Vpu (Cocka and Bates, 2012). Simultaneous expression of Tetherin and other viral antagonists can be used to study Tetherin-mediated restriction on viral budding.
Keywords: HIV-1Materials and Reagents
- HEK 293T cell line (ATCC, catalog number: CRL-11268 )
- Antibody, HIV-1 p24 Gag (NIH AIDS Reagent Program, catalog number: 24-3 )
- Antibody, HIV-1 Vpu (optional) (NIH AIDS Reagent Program, catalog number: 969 )
- Antibody, human Tetherin (optional) (NIH AIDS Reagent Program, catalog number: 11721 )
- Bromophenol Blue (Sigma-Aldrich, catalog number: B-5525 )
- Complete Mini protease inhibitor cocktail (F. Hoffmann-La Roche, catalog number: 04693132001 )
- Criterion Precast Gels, 4-15% Tris-HCl (Bio-Rad Laboratories, catalog number: 345-0027 )
- Dithiothreitol (DTT) (Thermo Fisher Scientific, catalog number: BP172-5 )
- Dulbecco’s Modified Eagle Media (DMEM) (Life Technologies, InvitrogenTM, catalog number: 11965-084 )
- Dulbecco’s Phosphate-buffered saline (DPBS) (Life Technologies, InvitrogenTM, catalog number: 14190-136 )
- 0.5 M EDTA solution (Life Technologies, InvitrogenTM, catalog number: 15575-038 )
- Fetal Bovine Serum (FBS) (Sigma-Aldrich, catalog number: F2442-500ML )
- Glycerol (Thermo Fisher Scientific, catalog number: BP229-1 )
- Hydrochloric acid (HCl) (Thermo Fisher Scientific, catalog number: A144-212 )
- Instant Nonfat Dry Milk (Nestle, catalog number: 050000033188 )
- Lipofectamine 2000 (Life Technologies, InvitrogenTM, catalog number: 11668-019 )
- NaCl (Thermo Fisher Scientific, catalog number: BP 358-212 )
- Opti-MEM (Life Technologies, InvitrogenTM, catalog number: 31985-070 )
- Plasmid, human Tetherin cDNA (optional) (OpenBiosystems, catalog number: 5217945 )
- Plasmid, psPAX2 HIV Gag (Addgene, catalog number: 12260 )
- Plasmid, Tetherin antagonist (HIV-1 Vpu) (optional)
- Sodium dodecyl sulfate (SDS) (Thermo Fisher Scientific, catalog number: BP166-500 )
- D-Sucrose (Thermo Fisher Scientific, catalog number: BP220-1 )
- Tris Base (Thermo Fisher Scientific, catalog number: BP152-500 )
- Triton X-100 (Thermo Fisher Scientific, catalog number: BP151-500 )
- Tween 20 Thermo Fisher Scientific, catalog number: BP337-500 )
- Cell culture media (see Recipes)
- 20% sucrose solution (see Recipes)
- Triton X-100 lysis buffer (see Recipes)
- Blocking solution (see Recipes)
- 6x protein gel loading buffer (see Recipes)
Equipment
- 24 well plate, cell culture treated (Corning, catalog number: 3524 )
- 1.7 ml Microcentrifuge tubes (GeneMate, catalog number: C-3260-1X )
- 8 x 34 mm Ultracentrifuge tubes (Beckman Coulter, catalog number: 343776 )
- Tabletop Centrifuge, refrigerated (Eppendorf Centrifuge, model: 5417R )
- TLA 120.1 rotor (Beckman Coulter, model: 357655 )
- Ultracentrifuge, refrigerated (Beckman Coulter Optima, model: TLX-IM-3 )
Procedure
- Plate 293T cells in 500 μl of Cell Culture Media at a concentration of 2.5 x 105 cells/well in a 24 well plate. Cells should grow to 70-80% confluence 18-24 h post-seeding.
- For the following transfection, the amounts of plasmid DNA have been optimized for this protocol. Transfect seeded cells with 50 ng of psPAX2, an HIV gag expression vector, using Lipofectamine 2000. Follow the manufacturer’s protocol using a ratio of Lipofectamine (μl):DNA (μg) of 2.5:1 diluted in OPTI-MEM. Aspirate the transfection reagent from the transfected cells 5 hours post transfection and add 500 μl fresh Cell Culture Media. Incubate at 37 °C and 5% CO2 for 36-48 h.
- Alternatively cells can be transfected with any other vector expressing a viral matrix/capsid protein, such as Ebola VP40 (Kaletsky, 2009) that can produce virus-like particles (VLPs).
- The effect of the cellular restriction factor Tetherin can be assessed by co-transfecting increasing amounts (10-200 ng) of the pCMV-SPORT6 Tetherin expression vector with a VLP producing plasmid.
- Viral antagonists of Tetherin can be studied by co-transfection of increasing amounts (25-100 ng) of a Tetherin antagonist such as pCAGGS HIV-1 Vpu along with a Tetherin and VLP expression plasmid.
- Alternatively cells can be transfected with any other vector expressing a viral matrix/capsid protein, such as Ebola VP40 (Kaletsky, 2009) that can produce virus-like particles (VLPs).
- Harvest cellular proteins and purify VLPs.
- VLP analysis. 36-48 h post-trasnfection, pipet all of the media from each transfected well into individual 1.7 ml microcentrifuge tubes. Centrifuge at 1,700 x g for 2 min at 4 °C to clear media and pellet cell debris. Transfer 450 μl of cleared media by pipetting into individual 8 x 34 mm ultracentrifuge tubes and underlay with 100 μl of the 20% Sucrose Solution. Place ultracentrifuge tubes in a pre-chilled TLA 120.1 rotor and centrifuge at 40,000 rpm for 30 min at 4 °C in a refrigerated ultracentrifuge. Carefully remove and discard all of the media and sucrose underlay by pipetting. Be very careful, as the protein pellet is not clearly visible. Add 50 μl PBS to the protein pellet on ice and incubate for 1-24 h (1 h minimum).
- Cellular Lysate Analysis. After removing the VLP containing media, add 80 μl Triton X-100 Lysis Buffer to each transfected well in the 24-well plate and incubate for 5 min at room temperature. Transfer all of the lysate to a 1.7 ml microcentrifuge tube. Centrifuge at 17,900 x g for 3 min at 4 °C to pellet insoluble cell debris. Transfer 50 μl of the cleared lysate to new microcentrifuge tube for immunoblot analysis.
- VLP analysis. 36-48 h post-trasnfection, pipet all of the media from each transfected well into individual 1.7 ml microcentrifuge tubes. Centrifuge at 1,700 x g for 2 min at 4 °C to clear media and pellet cell debris. Transfer 450 μl of cleared media by pipetting into individual 8 x 34 mm ultracentrifuge tubes and underlay with 100 μl of the 20% Sucrose Solution. Place ultracentrifuge tubes in a pre-chilled TLA 120.1 rotor and centrifuge at 40,000 rpm for 30 min at 4 °C in a refrigerated ultracentrifuge. Carefully remove and discard all of the media and sucrose underlay by pipetting. Be very careful, as the protein pellet is not clearly visible. Add 50 μl PBS to the protein pellet on ice and incubate for 1-24 h (1 h minimum).
- Immunoblot Analysis. For each sample, either sucrose pelleted VLPs or cellular lysates, transfer 25 μl into a new 1.7 ml microcentrifuge tube. Add 5 μl of 6x protein gel loading buffer to each sample. Heat the samples to 95 °C for 10 min. Analyze the VLPs and cellular lysates on separate Criterion Precast 4-15% SDS-PAGE gels. Transfer gel to Western blot membrane and continue to immunoblot using the Blocking Solution. Immunoblot with an anti-p24 Gag antibody (1:10,000 dilution in Blocking Solution) to detect the VLPs and cellular p24 gag. Membranes can also be stripped and immunoblotted for other transfected proteins (Tetherin, Vpu) or housekeeping genes as loading controls (GAPDH). For example, Tetherin can be used to restrict VLP release as shown in Figure 1. Samples containing VLPs co-transfected with Tetherin have less detectable p24 Gag expression compared to VLPs co-transfected without Tetherin. In contrast, the cellular lysates exhibit detectable p24 Gag across all samples, irrespective of Tetherin expression. Rescue of VLP release can be observed in samples expressing increasing amounts of a Tetherin antagonist.
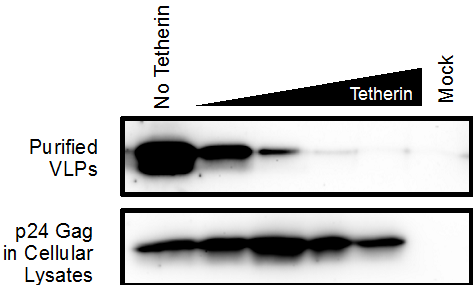
Figure 1. An immunoblot showing the effects of Tetherin on p24 Gag VLP production and release. In the top image, release of VLPs into the culture media is significantly decreased in the presence of Tetherin. However, production of p24 Gag in the cellular lysates is unaffected by increased Tetherin expression.
Recipes
- Cell Culture Media (1 L solution)
1 L DMEM
10% FBS - 20% sucrose solution (100 ml solution)
20 g sucrose
100 ml DPBS - Triton X-100 lysis buffer
50 mM Tris-HCl
150 mM NaCl
Buffer the solution to pH 7.5 with HCl
5 mM EDTA
1% Triton X-100
Immediately prior to use, add complete Mini protease inhibitor to Triton X-100 Buffer at concentration suggested by manufacturer. - Blocking Solution (1 L solution)
50 mM Tris-HCl
150 mM NaCl
Buffer the solution to pH 7.5 with HCl
50 g Instant Nonfat Dry Milk
1 ml Tween-20 - 6x protein gel loading buffer
350 mM Tris-HCl buffered to pH 6.8
0.6 M DTT
10% SDS
30% glycerol
0.012% Bromophenol Blue
Acknowledgments
This protocol was adapted from and recently used in Cocka and Bates (2012).
References
- Cocka, L. J. and Bates, P. (2012). Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog 8(9): e1002931.
- Kaletsky, R. L., Francica, J. R., Agrawal-Gamse, C. and Bates, P. (2009). Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A 106(8): 2886-2891.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Vande Burgt, N. H., Cocka, L. J. and Bates, P. (2013). HIV-1 Virus-like Particle Budding Assay. Bio-protocol 3(14): e830. DOI: 10.21769/BioProtoc.830.
Category
Microbiology > Microbial biochemistry > Protein > Immunodetection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link