- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Scanning Electron Microscope (SEM) Imaging to Determine Inflorescence Initiation and Development in Olive
Published: Vol 7, Iss 19, Oct 5, 2017 DOI: 10.21769/BioProtoc.2575 Views: 8965
Reviewed by: Scott A M McAdamAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Modified Pseudo-Schiff Propidium Iodide for Staining the Shoot Apical Meristem in Arabidopsis
Ruiqi Li [...] Ligeng Ma
May 5, 2023 2273 Views

A Novel Imaging Protocol for Investigating Arabidopsis thaliana Siliques and Seeds Using X-rays
Brylie A. Ritchie [...] Ansul Lokdarshi
Oct 5, 2023 2189 Views

Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Pradeep Chand Deo
Oct 20, 2025 1438 Views
Abstract
Here we present a protocol that describes how to image the structure of the olive axillary bud meristem with a scanning electron microscope (SEM) in order to characterize its identity and developmental stage. Briefly, the specimen is fixed with glutaraldehyde, saturated with ethanol, dried in a critical point dryer (CPD) system, dissected, coated with a conducting material and imaged with a scanning electron microscopy (SEM).
Keywords: SEMBackground
The exact timing of flowering induction and inflorescence initiation in olive (Olea europaea L.) is in controversy (Haberman et al., 2017). In olive, inflorescences emerge from lateral buds at the end of winter and flower in the spring. We have developed a protocol to better characterize the timing of inflorescence initiation in olive by imaging the meristem in the olive bud with a SEM at different times during the year. In these SEM images the meristem structure can be identified unambiguously, and the definition level of the meristem can be much higher than images of bud meristem sections presented in previous studies.
Materials and Reagents
- Scalpel blade No. 11 (Sigma-Aldrich, catalog number: S2771 )
- Double-sided adhesive tape
- Glass scintillation vials with screw caps, volume 20 ml (Sigma-Aldrich, catalog number: Z190535 )
- Pipette (BRAND, catalog number: 747760 ) or a similar instrument
- Gold annular target for the sputter coater (Agar scientific, catalog number: AGB7370 )
- Olive (Olea europaea L.) buds
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S3264 )
- Sodium phosphate monobasic (NaH2PO4) (Sigma-Aldrich, catalog number: S3139 )
- 25% glutaraldehyde (Sigma-Aldrich, catalog number: G5882 )
- Ethanol absolute (Sigma-Aldrich, catalog number: 24102 )
- Optional: Technical grade ethanol (Sigma-Aldrich, catalog number: V0T0042 )
- 0.1 M phosphate buffer pH 7.2 (sodium phosphate buffer; see Recipes)
- 5% glutaraldehyde solution (in 0.1 M phosphate buffer pH 7.2; see Recipes)
Equipment
- Scalpel handle No. 3 (Sigma-Aldrich, catalog number: S2896 )
- Tweezers style #5 (Sigma-Aldrich, catalog number: T4537 )
- Critical point dryer system (BAL-TEC, model: CPD 030 )
- Stereo-microscope (Olympus, model: SZX12 )
- Sputter coater (E510 scanning electron microscope coating unit) (Polaron Instruments, model: E510 )
- Scanning electron microscope (JEOL, model: JSM-5410 LV )
Procedure
- Using a scalpel, separate the axillary buds from the shoot. Leave a portion from the stem connected to the bud for gripping the bud with tweezers. Cut the opposite side to the bud of the stem piece vertically so that you can place the sample with the bud facing up (see Figure 1B).
- Add 5-10 ml (enough to cover the buds) of the 5% glutaraldehyde solution (see Recipes) to a scintillation vial. Immerse the buds in the glutaraldehyde solution for 24 h at room temperature (fixative solution [Sabatini et al., 1963]; see Figure 1C).
- Use a pipette to remove the glutaraldehyde solution from the vial.
- Wash the buds by adding phosphate buffer (see Recipes) to the buds. After 10 min, remove the buffer. Repeat this wash step 5 times.
- Add 25% ethanol solution, suspend for 1 h and remove the solution.
- Add 50% ethanol solution, suspend for 1 h and remove the solution.
- Add 75% ethanol solution, suspend for 1 h (break point) and remove the solution.
Note: If desired the fixing procedure can be suspended at this step (75% ethanol solution). Buds can be stored in a 75% ethanol solution at 4 °C over-night or up to several weeks. - Add 95% ethanol solution, suspend for 1 h and remove the solution.
- Add 100% ethanol solution, suspend for 1 h and remove the solution.
- Add 100% ethanol solution.
- Dry the buds in a Critical point dryer (CPD) instrument according to manufacturer's instructions (the drying procedure should take about 2 h).
- Use tweezers to hold the dry bud by the stem piece. Using a stereo microscope, carefully remove the leaf primordia from the bud with a scalpel or tweezers (Style #5). Normally, after 4-5 pairs of leaf primordia are removed, the meristem is exposed.
Note: This is a crucial step, take the time to gently remove the leaf primordia without damaging the meristem. - Bond a piece of double-sided adhesive tape to a metal stub (the stub goes into the SEM; see Figures 1E and1F). Use tweezers to bind the stem piece of the dissected bud to the metal stub, with the exposed meristem facing upwards (see Figure 1G).
- Coat the specimen with gold (Au) or gold/palladium (Au/Pd) in a sputter coater instrument according to manufacturer's instructions (see Figures 1H and 1I).
*Coating parameters we used: vacuum 0.02 Mbar, sputtering voltage 2.4 KV, current 20 mA, coating time 150 sec.
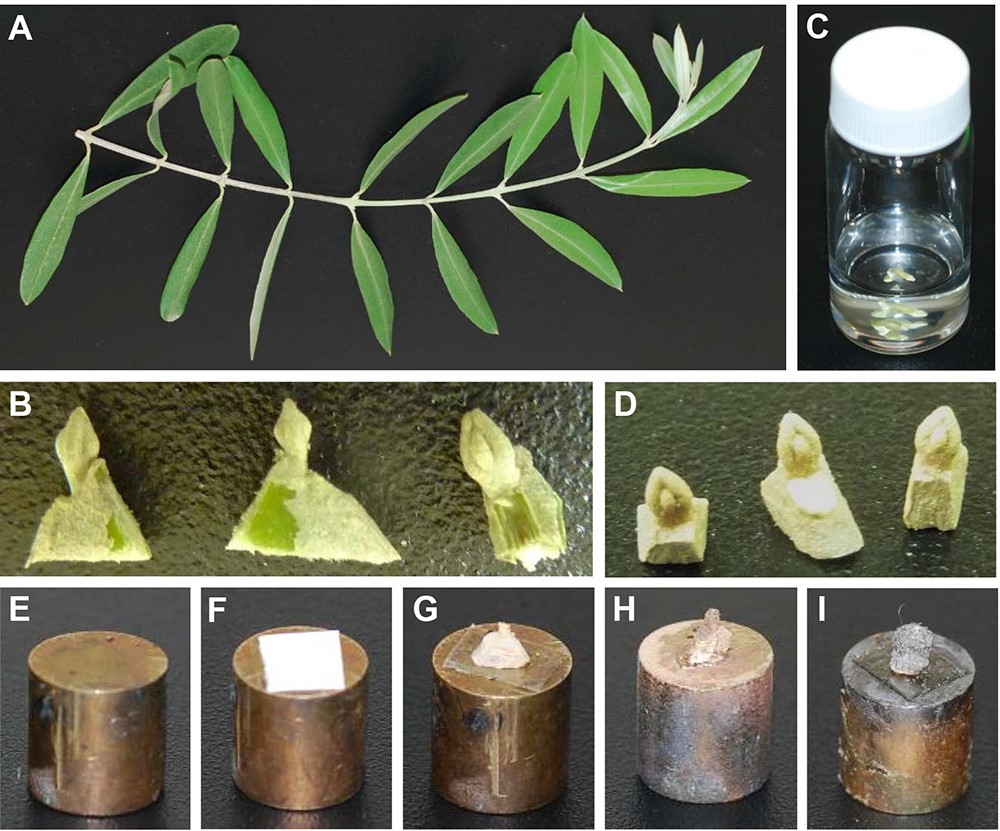
Figure 1. Preparation of the specimen for imaging. A. Picture of the olive shoot; B. Separated bud samples with attached stem pieces; C. Bud samples immersed in the fixative solution in a scintillation vial; D. Dried bud samples after drying in the CPD instrument; E. Metal stub used for the SEM imaging; F. Metal stub with a piece of double-sided adhesive tape; G. Dissected dried bud sample mounted on a metal stub; H. Dissected dried bud sample coated with gold; I. Dissected dried bud sample coated with gold-palladium. - Place the stub in the SEM and operate the SEM according to manufacturer's instructions to produce images of the meristem (see Figure 2).

Figure 2. SEM image of a whole specimen. Taken under high vacuum condition at accelerating voltage of 20 kV. Bud was sampled on 23 February 2011. Scale bar = 1 mm.
Data analysis
Crop the images to zoom and center the area of interest in the image, make sure you retain an accurate scale bar. Create a figure from the images you produced, using the software of your choice (see examples for figures created with PowerPoint [Microsoft] in Figures 3 and 4).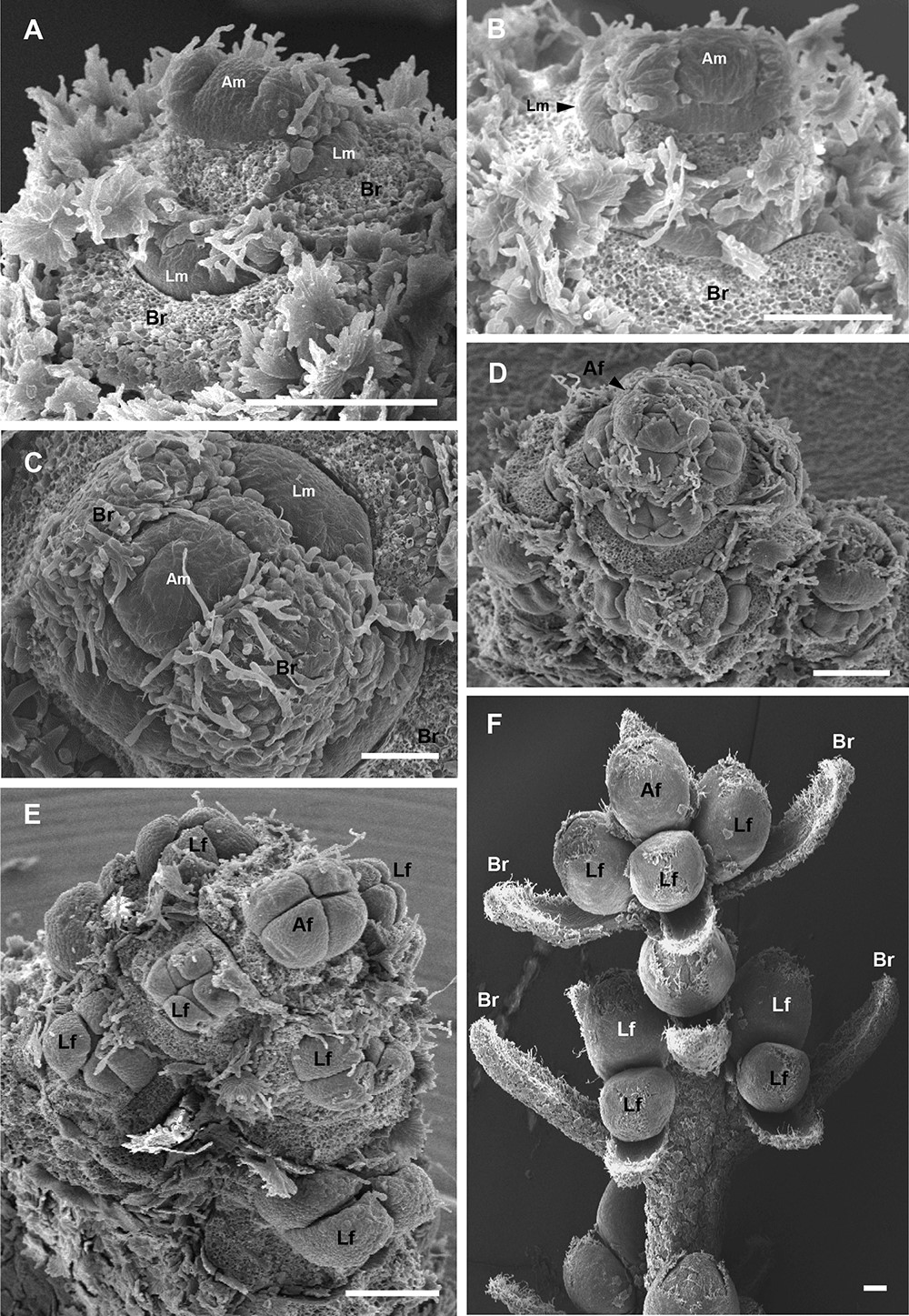
Figure 3. Development of inflorescence in Barnea olive. SEM images showing olive inflorescence at different stages of development. Images were produced from lateral buds sampled on 9 February 2011 (A), 16 February 2011 (B), 23 February 2011 (C-E) and 7 February 2010 (F). A-C. Initial stage of inflorescence development. The apical meristem (Am) do not initiate new leaf primordia, enlarge and bulge (‘dome’) subsequently becoming a terminal flower meristem. Lateral flower meristems (Lm) surrounding the Am begin bulging. Leaf primordia develop into bracts (Br). D-E. Subsequent stages of inflorescence meristem development. Apical flower meristem (Am) develops into apical flower (Af). Lateral flower meristems (Lm) differentiate into lateral flowers (Lf). F. Inflorescence before anthesis. Scale bar = 0.25 mm.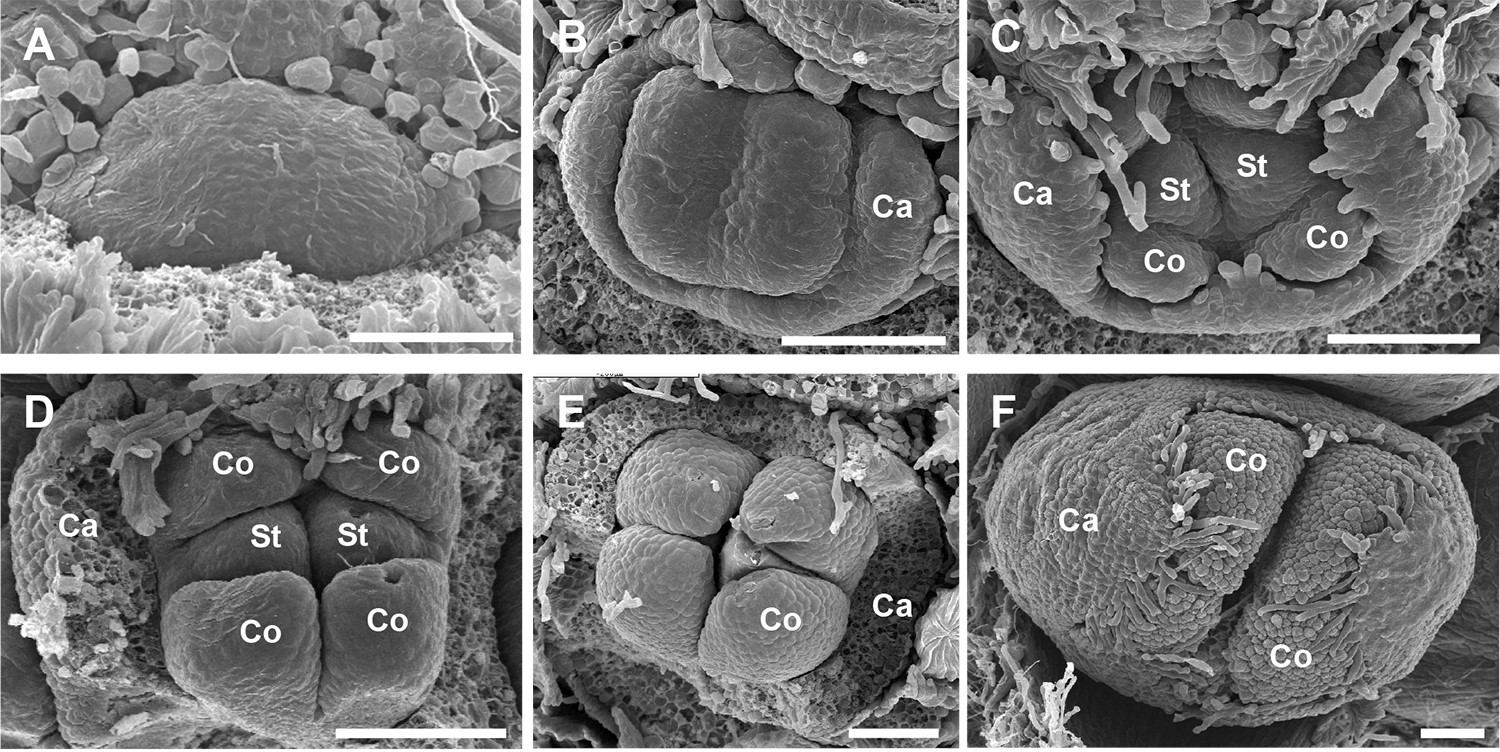
Figure 4. Development of Olive flowers. SEM images showing olive flowers at different stages of development. Images proudest from Barnea olive lateral buds sampled on 23 February 2011. A. Initial bulging (‘doming’) and enlargement of the meristem; B. The periphery of the meristem differentiates into the calyx (Ca; sepals); C-E. Subsequently, the central floral meristem develops into the corolla (Co) and two stamens (St), the pistil is not seen. F. Flower bud before anthesis. Scale bar = 0.1 mm.
Notes
- The diluted ethanol solutions can be prepared from cheaper technical grade ethanol (See Materials and Reagents #11).
- This protocol can be implemented in other plant species as demonstrated in apple (Malus domestica Borkh.; Haberman et al., 2016) and passion fruit (Passiflora edulis Sims; Nave et al., 2010).
Recipes
- 0.1 M sodium phosphate buffer pH 7.2 (1 L)
- First produce 1 M stock solutions of Na2HPO4 (dibasic) and NaH2PO4 (monobasic).
Dissolve 141.96 g of Na2HPO4 in distilled H2O and complete the final volume to 1 L. Do the same for 119.98 g of NaH2PO4. - Mix 68.4 ml of 1 M Na2HPO4 with 31.6 ml of 1 M NaH2PO4 and dilute to a final volume of 1 L. You get 1 L of 0.1 M sodium phosphate buffer pH 7.2.
- First produce 1 M stock solutions of Na2HPO4 (dibasic) and NaH2PO4 (monobasic).
- 5% glutaraldehyde solution in phosphate buffer
Dilute your glutaraldehyde solution according to its initial dilution in your 0.1 M phosphate buffer (Recipe 1). If you are using a stock solution of 25% glutaraldehyde, for a final volume of 100 ml, mix 20 ml of the 25% glutaraldehyde solution with 80 ml of the phosphate buffer.
Note: Glutaraldehyde is toxic and a strong irritant, always wear gloves, work in a chemical hood, dispose of it properly.
Acknowledgments
The fixation method in the protocol was composed according to the book, Fixation for electron microscopy (Hayat, 1981).
References
- Haberman, A., Ackerman, M., Crane, O., Kelner, J. J., Costes, E. and Samach, A. (2016). Different flowering response to various fruit loads in apple cultivars correlates with degree of transcript reaccumulation of a TFL1-encoding gene. Plant J 87(2): 161-173.
- Haberman, A., Bakhshian, O., Cerezo-Medina, S., Paltiel, J., Adler, C., Ben-Ari, G., Mercado, J. A., Pliego-Alfaro, F., Lavee, S. and Samach, A. (2017). A possible role for flowering locus T-encoding genes in interpreting environmental and internal cues affecting olive (Olea europaea L.) flower induction. Plant Cell Environ 40(8): 1263-128.
- Hayat, M. A. (1981). Fixation for electron microscopy. Academic Press.
- Nave, N., Katz, E., Chayut, N., Gazit, S. and Samach, A. (2010). Flower development in the passion fruit Passiflora edulis requires a photoperiod-induced systemic graft-transmissible signal. Plant Cell Environ 33(12): 2065-2083.
- Sabatini, D. D., Bensch, K. and Barrnett, R. J. (1963). Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol 17: 19-58.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Haberman, A., Zelinger, E. and Samach, A. (2017). Scanning Electron Microscope (SEM) Imaging to Determine Inflorescence Initiation and Development in Olive. Bio-protocol 7(19): e2575. DOI: 10.21769/BioProtoc.2575.
Category
Plant Science > Plant developmental biology > Morphogenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link


.jpg)







