- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Rice Root Organic Acid Efflux Measurement by Using Ion Chromatography
Published: Vol 7, Iss 4, Feb 20, 2017 DOI: 10.21769/BioProtoc.2141 Views: 9140
Reviewed by: Arsalan DaudiWan-Jun ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Heterologous Production of Artemisinin in Physcomitrium patens by Direct in vivo Assembly of Multiple DNA Fragments
Nur Kusaira Khairul Ikram [...] Henrik Toft Simonsen
Jul 20, 2023 2328 Views

Utilizing FRET-based Biosensors to Measure Cellular Phosphate Levels in Mycorrhizal Roots of Brachypodium distachyon
Shiqi Zhang [...] Maria J. Harrison
Jan 20, 2025 2380 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1171 Views
Abstract
Organic acids secreted from plant roots play important roles in various biological processes including nutrient acquisition, metal detoxification, and pathogen attraction. The secretion of organic acids may be affected by various conditions such as plant growth stage, nutrient deficiency, and abiotic stress. For example, when white lupin (Lupinus albus L.) is exposed to phosphorus (P)-deficient conditions, the secretion of citrate acid from its proteoid roots significantly increases (Neumann et al., 1999). This protocol describes a method for the collection and measurement of the efflux of organic acids (oxalate, malate, and citrate) from the roots of rice cultivar Nipponbare (‘Nip’) under different nitrogen forms (NH4+ and NO3-), together with different P supply (+P and -P) conditions.
Keywords: Organic acidsBackground
In addition to enzymatic methods (Delhaize et al., 1993) and high-performance liquid chromatography (HPLC) (Chen et al., 2013), ion chromatography is another widely used method for the determination of organic acids, which has previously been employed to detect the significant increase in oxalate content in taro root exudates during Al3+ stress (Ma and Miyasaka, 1998). Compared to ion chromatography, alternative methods have their own defects. For example, enzymatic methods require the use of enzymes that can easily undergo denaturation. Moreover, it is difficult to distinguish oxalate acid from Cl- peaks by HPLC. Here, we describe a method for analyzing organic acids secreted by rice roots using ion chromatography. This method could be used in the analysis of organic acids that are secreted by other hydroponically cultivated plants.
Materials and Reagents
- 0.2 µm syringe filter (Beyotime, catalog number: FF252 )
- Rice seedlings
- Nutrient solution
- Amerlite IR-120B resin (H+ form) (Alfa Aesar, catalog number: L14285 )
- Dowex 1 x 8 resin (100-200 mesh, formate form)
- Distilled water
- Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 258148 )
- Formic acid (HCOOH) (Sigma-Aldrich, catalog number: 695076 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 221465 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: 223506 )
- Oxalate (Sigma-Aldrich, catalog number: 75688 )
- Malate (Sigma-Aldrich, catalog number: M8304 )
- Citrate (Sigma-Aldrich, catalog number: 251275 )
- 1 N HCl (see Recipes)
- 2 N HCl (see Recipes)
- 2 M HCOOH (see Recipes)
- 4 N NaOH (see Recipes)
- 0.5 mM CaCl2 (see Recipes)
Equipment
- 1.25 L black plastic pot (plastic pot must be opaque, if required, wrap it in black tape)
- pH meter (Mettler Toledo, model: S40 / SG78 / SG23 / SG68 )
- Cation exchange column (16 mm x 14 cm) (Bio-Rad Laboratories, model: 732-1010 )
- Rotary evaporator (IKA, model: RV10 )
- Ion chromatography (Thermo Fisher Scientific, Dionex, model: ICS 3000 System )
- IonPac AS11 anion-exchange analytical column (4 x 250 mm)
- Guard column (4 x 50 mm)
Procedure
- Cultivate rice seedlings in full strength nutrient solution for 7 d. Afterwards, transplant 10 uniform seedlings to 1.5-L pots. After 6 d of treatments, discard the nutrient solution, and treat these rice seedlings with 0.5 mM CaCl2 (pH 5.5) overnight to collect the organic acids (Figure 1).

Figure 1. The apparatus for collection of root exudates - After 12 h, collect and weigh the fresh roots of the treated rice seedlings. Pass the root exudates in the 0.5 mM CaCl2 solution through a cation exchange column (16 mm x 14 cm) that contained 5 g of Amerlite IR-120B resin (H+ form) and an anion exchange column filled with 1.5 g of Dowex 1 x 8 resin (100-200 mesh, formate form) (Figure 2). Overall, this process takes approximately about 5.5 h.
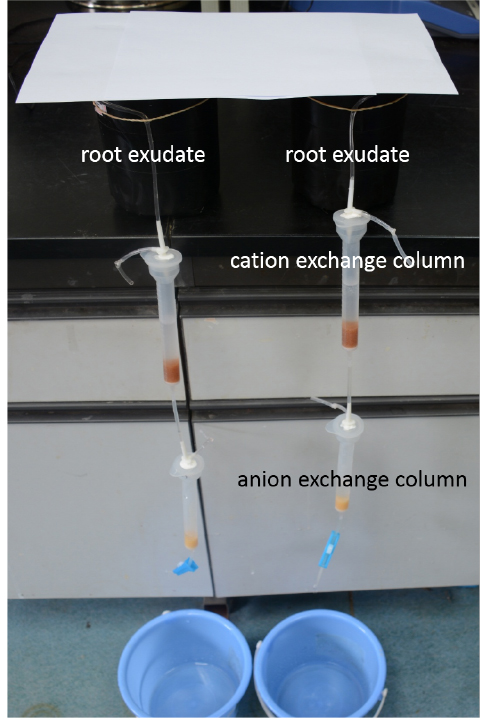
Figure 2. The equipment for retention of the organic acids - Elute the organic acid anions that were retained in the anion exchange resin immediately after the second step using 15 ml of 1 N HCl (Figure 3).
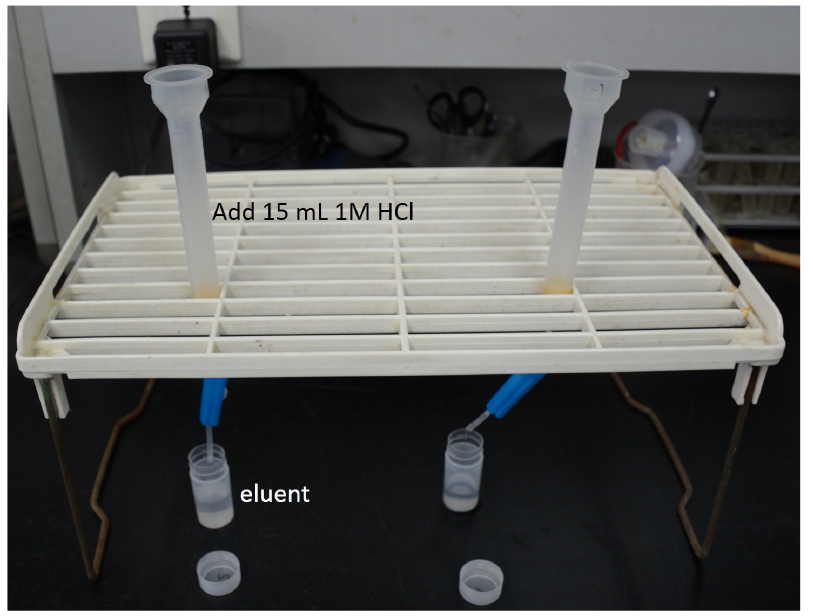
Figure 3. The equipment for eluting the organic acids - Then, evaporate the eluent by using a rotary evaporator at 40 °C, a rotation speed of 120 rpm, and a vacuum pressure of 35 hPa (Figure 4).

Figure 4. The equipment used in concentrating the organic acids - Terminate the evaporation process when the eluent solution has completely dried into powder (approximately 30 min later). Then re-dissolve the powder in 1 ml of distilled water and filter the solution through a 0.2 µm syringe filter prior to analysis.
- Detect the organic acid anions by ion chromatography (ICS 3000; Dionex) equipped with an IonPac AS11 anion-exchange analytical column (4 x 250 mm) and a guard column (4 x 50 mm). The mobile phase is 1.2 g/L NaOH at a flow rate of 0.6 ml/min. Generate a standard curve by using 1, 5, 10, 30, 50, 80, and 100 mg/L of the organic acid (oxalate, malate, and citrate) stock solution.
- Calculate the organic acid efflux expressing it as a function of the root fresh weight.
Notes:
- The recovery rate of organic acids must be > 80%. To calculate the recovery rate, at least two different concentrations of an organic acid (oxalate, malate, and citrate) stock solution were employed. For example, mix 20 μl or 100 μl 100 mg/L of organic acid stock solution with 1 L of distilled water, and repeat the procedure from 1 to 5 (theoretical concentration in distilled water is 2 or 10 μg/ml). Then, repeat step 6 of procedure and calculate the actual organic acids concentration. Finally, calculate the recovery rate using the following equation:
Recovery rate = (Actual value/Theoretical value) x 100. - To prepare the anion exchange resin, wash the anion exchange resin powder (500 g) in 1 L 4 N NaOH for about 30 min at room temperature. Repeat three times and wash with distilled water to neutral pH. Wash three more times in 1 L 2 M formic acid for about 30 min each and wash again with distilled water to neutral pH.
Similarly, wash the cation exchange resin (500 g) four times with 0.5 L 4 N NaOH about 1 h at 60 °C each, then rinse it with distilled water to a neutral pH, afterwards, wash it three times in 0.5 L 2 N HCl about 1 h each at room temperature, then rinse it with distilled water to a neutral pH.
The prepared resins can be stored at 4 °C for at least 1 year. - This method can be easily adapted to other plant species such as Arabidopsis (Zhu et al., 2012). However, the secretion of organic acids is limited for some small plant species. In this case, the recovery rate can be relatively low, and thus the number of plants per pot must be scaled up in order to collect enough organic acids.
Data analysis
- Create a standard curve for each organic acid by plotting the peak area vs. the known concentration.
- Use the standard curves to calculate the concentrations of each organic acid. This will give the concentration of organic acid present in the eluent. Data can be divided by root fresh weight to determine the amount of organic acids secreted per grams of rice root.
The concentration of organic acids was calculated using the following formula:
F= C x V/W
Where,
F is the organic acid secreted in roots (μg/g Fw),
C is the concentration of organic acid (mg/L),
V is the volume of the organic acid (this is 1 ml in the present study),
W is the fresh weigh of 10 rice roots in one pot (g Fw)
Below is an example of a calculation for the concentration of malate. The area of the sample is 0.0612, which was then used in the standard curve equation y = 0.0084x + 0.0056 (Figure 5), which calculated that the concentration of malate is 6.6190 mg/L. The volume of malate in present study was 1 ml, whereas the root fresh weight was 0.6126 g. The obtained value was then used in the above mentioned formula to calculate for the concentration in the roots (10.8048 μg/g Fw).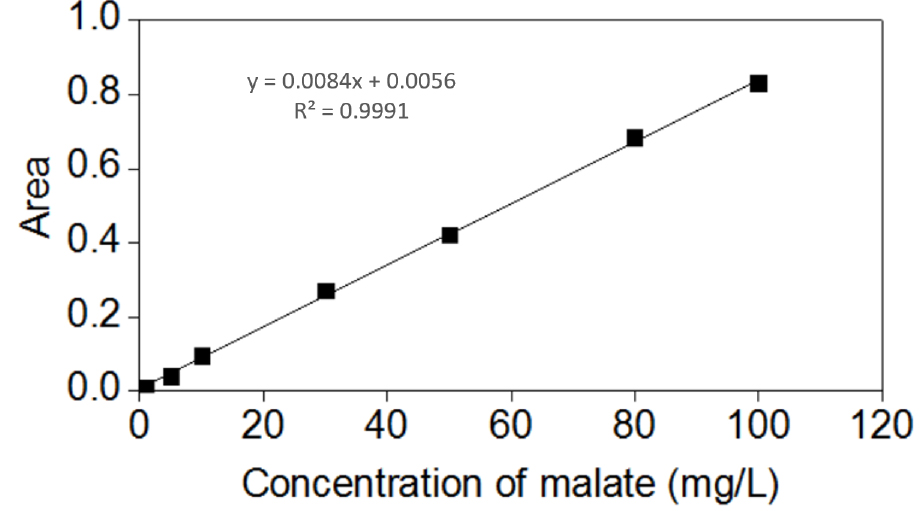
Figure 5. Standard curve of malate
Recipes
- 1 N HCl
Add ~88 ml 37% HCl to about 500 ml of distilled water, then adjust the final volume of the solution to 1 L using distilled water - 2 N HCl
Add ~176 ml 37% HCl to about 500 ml of distilled water, then adjust the final volume of the solution to 1 L using distilled water - 2 M formic acid (HCOOH)
Add ~78.6 ml 96% HCOOH to about 500 ml of distilled water, then adjust the final volume of the solution to 1 L using distilled water - 4 N NaOH
Add 166.7 g NaOH to distilled water to a final volume of 1 L - 0.5 mM CaCl2 (pH 5.5)
- 0.5 M CaCl2 stock solution
Dissolve 73.505 g CaCl2·2H2O in distilled water and add distilled water to a final volume of 1 L - 0.5 mM CaCl2
Dilute 5 ml of 0.5 M CaCl2 stock solution with distilled water to a final volume of 5 L
Adjust the pH of 0.5 mM CaCl2 solution to 5.5 with 1 N HCl - 100 mg/L organic acid stock solution (oxalate, malate and citrate)
100 mg oxalate, malate, and citrate together
Add distilled water to 1 L to make a 100 mg/L stock solution
Dilute the stock solution to 1, 5, 10, 30, 50, 80, and 100 mg/L to generate a standard curve
Acknowledgments
The methods to collect and concentrate organic acids were modified from Ma et al. (2004), the measurement of organic acids content was modified from Zhu et al. (2015) and developed in the Ren Fang Shen’s Lab-Institute of Soil Science, Chinese Academy of Science. This work was supported by the National Key Basic Research Program of China (No. 2014CB441000), Natural Science Foundation of China (31501825) and the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (Nos. XDB15030302 and XDB15030202).
References
- Chen, J., Wan, W. H., Wu, F. H., You, C. Y., Liu, T. W., Dong, X. J., He, J. X. and Zheng, H. L. (2013). Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362: 301-318.
- Delhaize, E., Ryan, P. R. and Randall, P. J. (1993). Aluminum Tolerance in Wheat (Triticum aestivum L.) (II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiol 103(3): 695-702.
- Ma, J. F., Nagao, S., Sato, K., Ito, H., Furukawa, J. and Takeda, K. (2004). Molecular mapping of a gene responsible for Al-activated secretion of citrate in barley. J Exp Bot 55(401): 1335-1341.
- Ma, Z. and Miyasaka, S. C. (1998). Oxalate exudation by taro in response to Al. Plant Physiol 118(3): 861-865.
- Neumann, G., Massonneau, A., Martinoia, E. and Römheld, V. (1999). Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208(3): 373-382.
- Zhu, X. F., Lei, G. J., Jiang, T., Liu, Y., Li, G. X. and Zheng, S. J. (2012). Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 236(4): 989-997.
- Zhu, X. F., Wang, Z. W., Wan, J. X., Sun, Y., Wu, Y. R., Li, G. X., Shen, R. F. and Zheng, S. J. (2015). Pectin enhances rice (Oryza sativa) root phosphorus remobilization. J Exp Bot 66(3): 1017-1024.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhu, C., Zhu, X. and Shen, R. (2017). Rice Root Organic Acid Efflux Measurement by Using Ion Chromatography. Bio-protocol 7(4): e2141. DOI: 10.21769/BioProtoc.2141.
- Zhu, C. Q., Zhu, X. F., Hu, A. Y., Wang, C., Wang, B., Dong, X. Y. and Shen, R. F. (2016). Differential Effects of Nitrogen Forms on Cell Wall Phosphorus Remobilization Are Mediated by Nitric Oxide, Pectin Content, and Phosphate Transporter Expression. Plant Physiol 171(2): 1407-1417.
Category
Plant Science > Plant metabolism > Other compound
Plant Science > Plant biochemistry > Other compound
Biochemistry > Other compound > Acid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












