- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determining Efficiency and Selectivity of Lipid Extraction by Perturbing Agents from Model Membranes
Published: Vol 6, Iss 22, Nov 20, 2016 DOI: 10.21769/BioProtoc.2016 Views: 8159
Reviewed by: Marc-Antoine SaniVenkatasalam ShanmugabalajiDaniel Kraus

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Analysis and Quantification of the Mitochondrial–ER Lipidome
Alexis Diaz-Vegas [...] James G. Burchfield
Jul 5, 2024 2587 Views

Cost-Effective and Reproducible Preparation of mRNA-Loaded Lipid Nanoparticles Using a Conventional Laboratory-Scale Microfluidic Assembly System
Yunqi Li [...] Ruoyang Zhao
Sep 20, 2025 1452 Views

Lipid-Mediated Sequential Recruitment of Proteins Via Dual SLIPT and Dual SLIPTNVOC in Live Cells
Kristina V. Bayer and Richard Wombacher
Nov 5, 2025 1603 Views
Abstract
Several membrane-perturbing agents extract lipids from membranes and, in some cases, this lipid efflux is lipid specific. In order to gain a better description of this phenomenon and to detail the intermolecular interactions that are involved, a method has been developed to characterize the extent and the specificity of membrane-lipid extraction by perturbing agents. A perturbing agent is incubated with model membranes existing as multilamellar vesicles (MLVs) and subsequently, the remaining MLVs and the small lipid/perturbing agent complexes resulting from the extraction are isolated and analysed to assess the extent and the specificity of the lipid extraction.
Background
Several membrane-perturbing agents extract lipids from membranes; these include proteins, peptides, and detergents. Several of these lipid extractions are fundamental processes in biology. In some cases, the process is lethal; this is the case for some antibacterials that extract lipids from bacterial membranes leading to the death of the cells (Bechinger, 2014; Schaefer et al., 2014). In other systems, this process is vital. For example, ApoA1 is a protein that binds to cells and extracts phospholipids and cholesterol to form nascent high density lipoproteins (HDL), a process critical for the reverse cholesterol transport and therefore playing a pivotal role in the control of atherosclerosis (Strömstedt et al., 2010). It has been shown that some of these lipid extractions are lipid specific; in other words, the lipid composition of the extracted fraction is different than that of the original membranes. It is expected that such specificity will be reported more often considering the recent progress of lipidomics. In general, the lipid specificity of the induced lipid efflux is poorly characterized despite the pivotal role it plays in biological processes. We have recently shown that selective lipid efflux depends on specific interactions with membranes (Therrien et al., 2013; 2016). In order to gain a better description of this emerging phenomenon and to detail the intermolecular interactions that are involved, a method has been developed to characterize the extent and the specificity of membrane-lipid extraction by perturbing agents using model membranes. A perturbing agent is incubated with model membranes existing as multilamellar vesicles (MLVs). The classes of perturbing agents include proteins (e.g., binder-of-sperm proteins BSPA1, ApoA1), peptides (e.g., melittin, antibacterial peptides), and detergents (e.g., Triton X-100). The use of model membranes allows a complete control of the lipid composition, modulating in a precise manner molecular features such as the charge and the H-bond capacity, and defining how these factors contribute to the lipid specificity of the extraction. Subsequently, the remaining MLVs, which may include some perturbing agent, are separated by centrifugation from the small lipid/perturbing agent complexes resulting from the lipid extraction. The lipid compositions of the pellet (representative of intact membranes) and of the supernatant (representative of the lipid efflux) are analysed in order to assess the extent and the specificity of the lipid extraction.
Materials and Reagents
- Progene® 1.5-ml microcentrifuge tubes
- V-Vial screw-thread sample vials, 5 ml, with PTFE-lined caps
- Pyrex Brand 9800 test tubes, 20 ml
- Syringe
- Glass test tube
- Marbles
- YMC diol column
- Lipids (high purity) (Avanti Polar Lipids) (ex. 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine [POPC]; 1-palmitoyl-2-oleoyl-sn-glycero-phosphoethanolamine [POPE])
- Membrane perturbing agent of interest
- Benzene (ACS grade)
- Methanol (HPLC grade)
- Milli-Q water
- 3-[N-morpholino]propanesulfonic acid (MOPS) (> 95%)
- Sodium chloride (NaCl, high-purity grade)
- Ethylenediamine tetraacetic acid (EDTA) (99.4-100.6%)
- Potassium phosphate, monobasic (KH2PO4) (dried at 105 °C under vacuum for at least 4 h prior to its use to ensure it is dry)
- Concentrated sulphuric acid (H2SO4) (ACS reagent, 95.0-98.0%)
- Peroxide (H2O2) (30%) (ACS reagent)
- Sodium metabisulfite (Na2S2O5) (ACS reagent, >97%)
- Ammonium molybdate tetrahydrate [(NH4)6Mo7O24·4H2O] (ACS reagent, 81.0-83.0%)
- Ascorbic acid (99%)
- Acetonitrile (HPLC grade)
- Ammonium acetate (CH3CO2NH4) (≥ 98%)
- Nitrogen
Equipment
- Microcentrifuge (Eppendorf, model: 5417 R ) equipped with a rotor (Eppendorf, model: f45-30-11 )
- UV-Vis spectrometer (Agilent Technologies, model: Cary 6000i )
- LC/MS system (Agilent Technologies, model: 1100 series system ) with a 1100 MSD mass spectrometer (Agilent Technologies, model: 1100 MSD )
- Water bath
Procedure
Typically, a series of experiments is planned for a given perturbing agent and model membranes, at different ratios of lipid to perturbing agent. Samples are carried out in triplicates. Stock solutions are prepared to perform one set of measurements. For a single measurement, between 300 and 800 nmoles of phospholipids are needed; the lipid amount is kept constant in all samples.
- Model membrane preparation
- To form model membranes from a binary mixture of lipids, individual lipids are first dissolved in a benzene/methanol mixture. Typically an individual lipid stock solution is prepared by weighting exactly ~25 mg of lipid and by adding the appropriate volume of benzene/methanol (90/10, v/v) solution to obtain 5 mg of lipid/ml. For more polar species, such as phosphatidylserine, and cholesterol sulphate, the proportion of methanol can be increased to 85/15 (v/v). The lipid organic solutions are prepared in V-Vial screw-thread sample vials, 5 ml, in order to limit solvent evaporation.
- Aliquots with appropriated volumes of lipid solutions are mixed to obtain the desired molar ratio. The absolute amount of lipid depends on the number of planned samples.
- The lipid solution is then frozen in liquid nitrogen and lyophilized for at least 16 h to make sure that all the organic solvent is removed.
- The lipid powder is hydrated in a MOPS buffer (50 mM) containing 100 mM NaCl and 100 µM EDTA, and adjusted to pH 7.4. Typically the appropriate buffer volume is added to provide a MLV stock suspension of 5 mM.
- The samples are submitted to 3 freeze-and-thaw cycles (from liquid nitrogen temperature to a temperature about 10 °C above the temperature of the gel-to-fluid phase transition). The sample is vortexed when the sample is at high temperature to ensure the proper hydration of the lipids.
Example: model membrane preparation
Membranes made of 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-phosphoethanolamine (POPE) with a POPC/POPE molar ratio of 60/40; sufficient lipid amount for 10 samples.
Molecular weight (POPC) = 760.07 g/mol; Molecular weight (POPE) = 718.01 g/mol
Organic solution: 5 mg of phospholipid/ml.
730 µl of POPC organic solution (3.65 mg; 4.80 µmol) were mixed with 460 µl of POPE organic solution (2.30 mg; 3.20 µmol).
After lyophilization, the resulting lipid powder (5.95 mg; 8.00 µmol) was hydrated with 1.6 ml of buffer to provide a MLV stock suspension of 5 mM.
- To form model membranes from a binary mixture of lipids, individual lipids are first dissolved in a benzene/methanol mixture. Typically an individual lipid stock solution is prepared by weighting exactly ~25 mg of lipid and by adding the appropriate volume of benzene/methanol (90/10, v/v) solution to obtain 5 mg of lipid/ml. For more polar species, such as phosphatidylserine, and cholesterol sulphate, the proportion of methanol can be increased to 85/15 (v/v). The lipid organic solutions are prepared in V-Vial screw-thread sample vials, 5 ml, in order to limit solvent evaporation.
- Lipid extraction
The lipid extraction is determined for a given membrane composition, a given temperature, and a given concentration of perturbing agent. All the samples, including the controls (i.e., without the perturbing agent), have the same final volume, and final lipid concentration.- Each sample is a combination of an aliquot of MLV suspension, an aliquot of perturbing agent solution, and an aliquot of buffer. The final concentration of lipid is set between 1.5 and 4 mM, and the concentration of perturbing agent depends on the desired lipid/perturbation agent molar ratio. The total volume should be between 250 and 1,500 µl. The appropriate volume of MLV stock suspension is pipetted in a 1.5 ml centrifuge tube. The aliquot of perturbing agent solution, prepared in the same buffer as the lipid suspension, is added to obtain the desired lipid/perturbing agent ratio. An aliquot of buffer is added to ensure that all samples have the same volume and the same lipid concentration (Table1).
Table 1 shows an example of the composition of a sample series to determine the efficiency and selectivity of lipid extraction induced by a detergent. In this case, the concentration of the lipid stock solution is 40 mM while the concentration of the detergent stock solution is 10 mM.
Table 1. Example of sample series: Lipid extraction by a detergent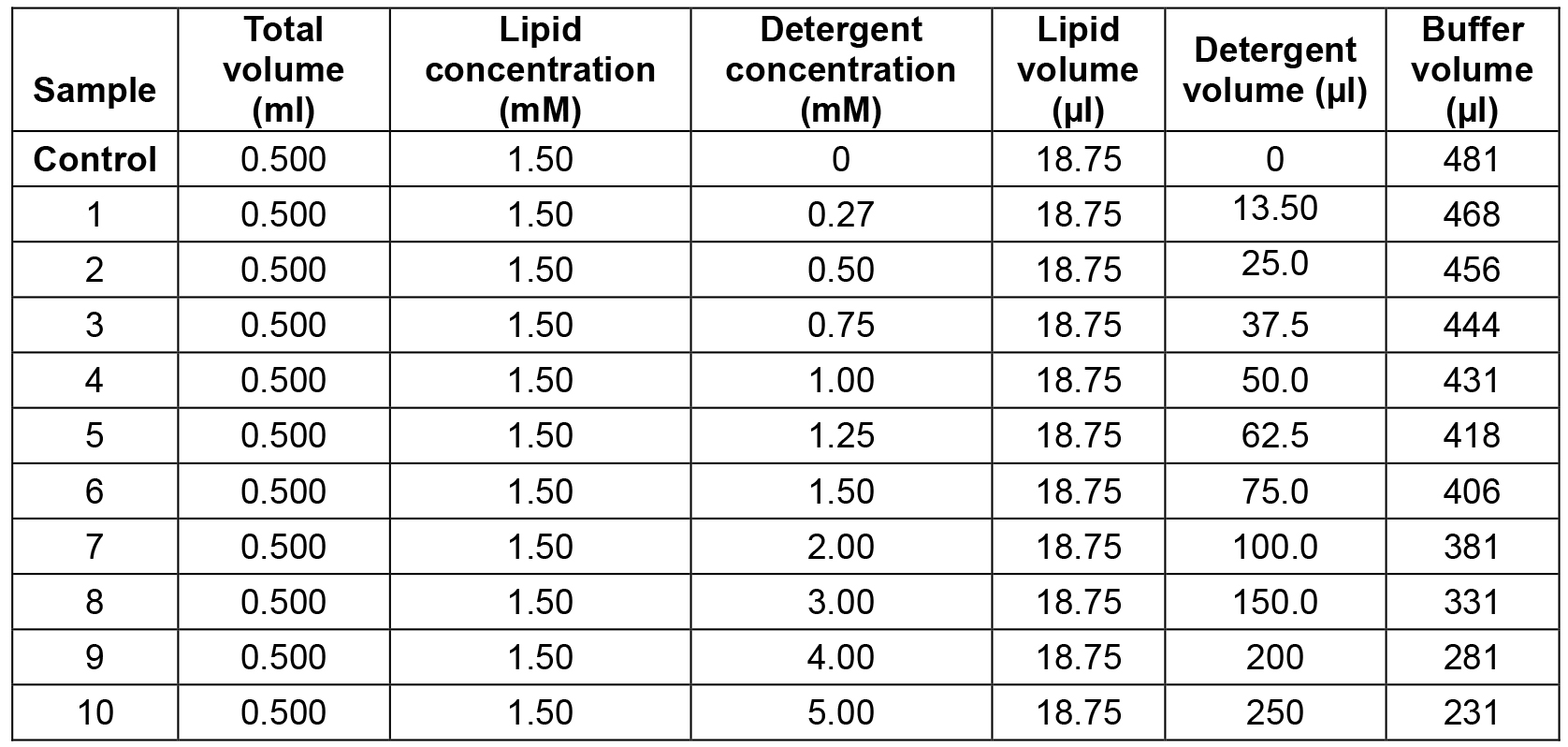
- The suspensions are then vortexed and incubated for at least 30 min at a given temperature, using a water bath.
- After the incubation, the samples are centrifuged for 5 min at 20,800 x g and 1 °C. The extracted lipids are defined as those existing in small perturbing agent/lipid assemblies that stay in the supernatant while the remaining MLVs (possibly with bound perturbing agent) pellet. Centrifugation of control samples (without perturbing agent) typically show that more than 95% of lipids are found in the pellets.
Note: Another temperature can be selected for the centrifugation; the control samples (without perturbing agent) provide a quantitative description of the efficiency of the separation of the extracted lipids from the remaining MLVs by centrifugation. - The supernatants are isolated using a syringe.
- The pellets are resuspended in 500 µl of MOPS buffer for their analysis.
- Each sample is a combination of an aliquot of MLV suspension, an aliquot of perturbing agent solution, and an aliquot of buffer. The final concentration of lipid is set between 1.5 and 4 mM, and the concentration of perturbing agent depends on the desired lipid/perturbation agent molar ratio. The total volume should be between 250 and 1,500 µl. The appropriate volume of MLV stock suspension is pipetted in a 1.5 ml centrifuge tube. The aliquot of perturbing agent solution, prepared in the same buffer as the lipid suspension, is added to obtain the desired lipid/perturbing agent ratio. An aliquot of buffer is added to ensure that all samples have the same volume and the same lipid concentration (Table1).
- Lipid analysis
Two parallel analyses are carried out to determine the extent of lipid extraction. First, the phospholipid contents in the supernatants, and in the pellets are determined by a Bartlett phosphorus assay (Bartlett, 1958). Second, an LC-MS analysis is carried out for the supernatants and the pellets. - Bartlett phosphorus assay
- An aliquot of the lipid sample containing between 8 and 70 nmol in phospholipid is transferred into a glass test tube. Typically, 20 µl of the supernatant and 20 µl of resuspended pellet are used.
- Standard samples containing between 0 (blank), and 3.5 mM phosphate are prepared in glass test tubes, from a KH2PO4 stock solution (3.5 mM in Milli-Q water; 47.63 mg in 100 ml). Typically, samples with 3.5, 2.0, 1.5, 1.0, 0.4 mM KH2PO4 are prepared.
- A 20-µl aliquot of each standard sample is transferred into a glass test tube.
- To each tube, 120 µl of concentrated sulphuric acid is added. The samples are then vortexed.
- To each tube, 20 µl of H2O2 (30%) is added. The samples are then vortexed.
- The tubes are heated all together to 200 °C for 10 min. During the heating, marbles are placed on top of the tubes to prevent potential evaporation.
- The samples are put on the bench and left to cool to room temperature.
- To each tube, 1,340 µl of Milli-Q water is added. The samples are then vortexed.
- To each tube, 40 µl of Na2S2O5 solution (526 mM in Milli-Q water; 100 mg in 1 ml) is added. The samples are then vortexed.
- The tubes are heated all together to ~90 °C for exactly 5 min.
- The samples are put on the bench and left to cool to room temperature.
- To each tube, 400 µl of (NH4)6Mo7O24·4H2O solution (16.2 mM in Milli-Q water; 200 mg in 10 ml) is added. The samples are then vortexed.
- To each tube, 40 µl of ascorbic acid (568 mM in Milli-Q water; 100 mg in 1 ml – the solution is photosensitive and must be kept in the dark prior its use) are added. The samples are then vortexed.
- The tubes are heated all together to ~90 °C for exactly 10 min. A blue color should appear.
- The samples are put on the bench and left to cool to room temperature.
- The absorbance at 820 nm is read within 1 h and is used for the phospholipid determination (Figure 1).
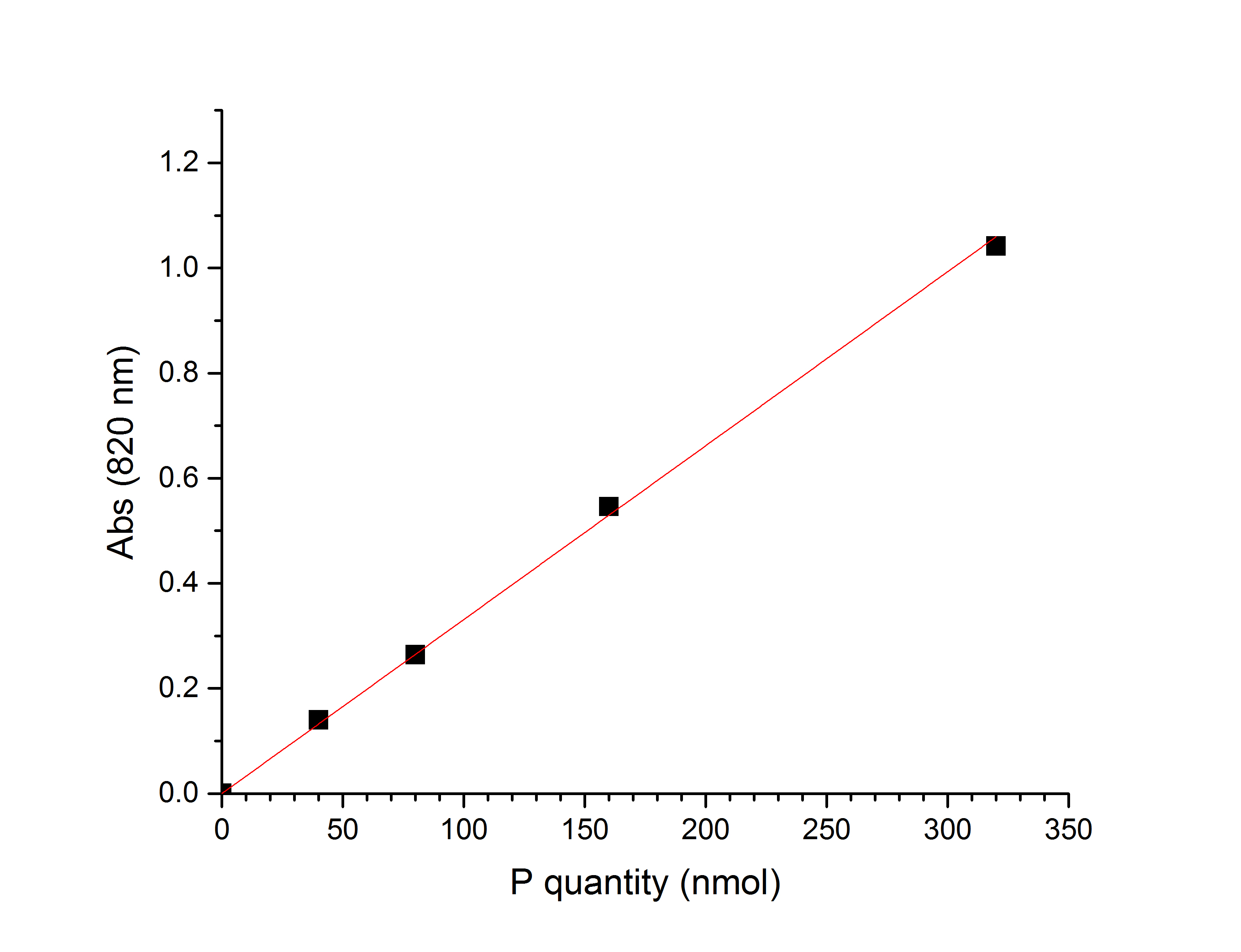
Figure 1. Typical calibration curve for the Bartlett assay
- An aliquot of the lipid sample containing between 8 and 70 nmol in phospholipid is transferred into a glass test tube. Typically, 20 µl of the supernatant and 20 µl of resuspended pellet are used.
- LC-MS analysis
The lipid composition of the supernatants and of the pellets are determined by LC-MS analysis. Typically 32-µl aliquots of each sample are injected and triplicates are carried out for each condition. Samples are eluted on a YMC diol column (4.6 x 150 mm, 5 µm particle size) (Agilent Technologies), maintained at 50 °C. Elution of the phospholipids is achieved in 7 min, using acetonitrile/aqueous ammonium acetate solution (100 mM) (85/15, v/v) at 0.6 ml/min. The ESI source is used in the positive ionization mode. Nitrogen is used as drying gas at 250 °C and 12 L/min. Nebulizing gas is also nitrogen, held at 241 kPa.- A 2-µl aliquot of the supernatant and of the resuspended pellet is diluted in 1 ml of an H2O/MeOH (1/9, v/v) solution.
- A 2-µl aliquot is injected for the LC/MS analysis.
- A 2-µl aliquot of the supernatant and of the resuspended pellet is diluted in 1 ml of an H2O/MeOH (1/9, v/v) solution.
Data analysis
- The Bartlett assay
The fraction of extracted phospholipids, f, is provided by: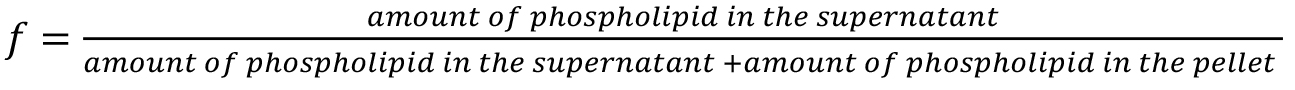
- The LC/MS analysis
The extent of extraction for each lipid species is determined using:
Extraction% = As/(As + Ap) x 100%
Where,
As and Ap are the lipid peak area from the supernatant and the pellet analysis, respectively. The lipid specificity of the extraction is obtained by comparing Extraction% calculated for each lipid. In the absence of specificity, Extraction% should be the same for the different lipids. A higher Extraction% factor for one lipid relative to the other defines its specific extraction.
Example: An example of results showing some lipid selectivity for a detergent-induced extraction of phosphatidylcholine (PC)/phosphatidylethanolamine (PE) membranes is presented in Table 2.
Table 2. Example of sample series: Selectivity of lipid extraction by a detergent. The lipid concentration was constant (1.5 mM) and the detergent concentration is varied.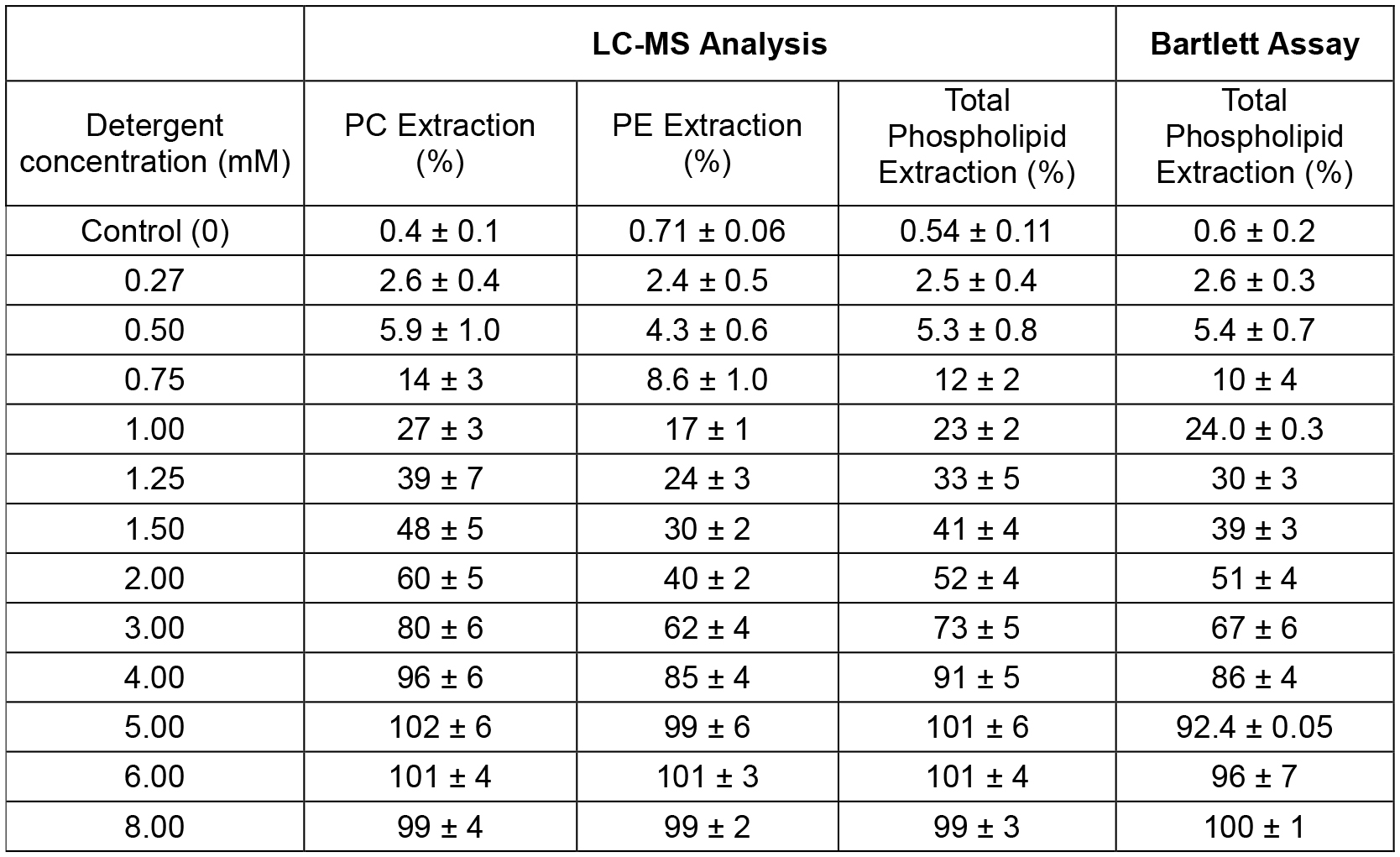
Notes
- For the Bartlett assay, the reproducibility for independent triplicates was typically 8% and better than 10%.
- For the LC/MS analysis, the reproducibility between the 3 injections of a sample is typically better than 1%, and it is better than 5% for independent triplicates.
- For detergent concentration between 0.75 and 4 mM, the extraction is PC specific as more PC are extracted relative to PE. When the MLVs are intact or completely disrupted, lipid extraction specificity cannot be expressed.
- The agreement of the total phospholipid extraction between the LC/MS and the Bartlett assay reflects the accuracy of the determination.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada, and by the Fonds de recherche du Québec-Nature et technologies through its Strategic Cluster program. The protocol is adapted from previous work (Therrien et al., 2013; 2016).
References
- Bartlett, G. R. (1958). Phosphorous assay in column chromatography. J Biol Chem 234: 466-468.
- Bechinger, B. (2014). The SMART model: Soft membranes adapt and respond, also transiently, in the presence of antimicrobial peptides. J Peptide Sci 21: 346-355.
- Schaefer, E. J., Anthanont, P. and Asztalos, B. F. (2014). High-density lipoprotein metabolism, composition, function, and deficiency. Curr Opin Lipidol 25:194-199.
- Strömstedt, A. A., Ringstad, L., Schmidtchen, A. and Malmsten, M. (2010). Interaction between amphiphilic peptides and phospholipid membranes. Curr Opin Colloids Interface Sci 15:467-478.
- Therrien, A., Fournier, A. and Lafleur, M. (2016). Role of the cationic C-terminal segment of melittin on membrane fragmentation. J Phys Chem B 120(17): 3993-4002.
- Therrien, A., Manjunath, P. and Lafleur, M. (2013). Chemical and physical requirements for lipid extraction by bovine binder of sperm BSP1. Biochim Biophys Acta 1828(2): 543-551.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lafleur, M. and Therrien, A. (2016). Determining Efficiency and Selectivity of Lipid Extraction by Perturbing Agents from Model Membranes. Bio-protocol 6(22): e2016. DOI: 10.21769/BioProtoc.2016.
Category
Biochemistry > Lipid > Lipid isolation
Biochemistry > Lipid > Lipid-protein interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











