- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of Acetylcholine from Cell Lines
Published: Vol 3, Iss 24, Dec 20, 2013 DOI: 10.21769/BioProtoc.1007 Views: 11202
Reviewed by: Lin FangFanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

FACS-based Glucose Uptake Assay of Mouse Embryonic Fibroblasts and Breast Cancer Cells Using 2-NBDG Probe
Shengli Dong and Suresh K Alahari
Apr 20, 2018 11556 Views

Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism
Birte Plitzko and Sandra Loesgen
May 20, 2018 76103 Views

Optimization of Extracellular Flux Assay to Measure Respiration of Anchorage-independent Tumor Cell Spheroids
Zaineb Javed [...] Nadine Hempel
Feb 20, 2022 4587 Views
Abstract
Cigarette smoking is the leading risk factor for the development of lung cancer. It is estimated that smoking is associated with 80-90% of lung cancer cases throughout the world (see References 1 and 2). The addictive component of cigarette smoke is nicotine. Our published data shows that nicotine promotes the production of acetylcholine (ACh) in human bronchioalveolar carcinoma cells (BACs) (Lau et al., 2013). ACh functions as a growth factor in human BACs. The following protocol is based on a published protocol by (Song et al., 2003), with some modifications (Lau et al., 2013; Song et al., 2008; Song et al., 2003; Sekhon et al., 2003). An important point to remember is that fetal bovine serum (FBS) contains a high amount of acetylcholine (ACh). Therefore, cells must be cultured in serum-free medium to measure ACh in the culture supernatant. Two aliquots of the culture supernatant are used for analysis. This protocol measures the total choline in the cell supernatent under two conditions: 1) After treatment with acetylcholinesterase (AChE), which converts the ACh to choline (also called the total choline sample) and 2) after measuring the amount of free choline in the sample. The concentration of ACh in the sample calculated by subtracting the free choline from the total choline.
Materials and Reagents
- A549 cells (American Type Culture Collection)
- Human Epidermal Growth Factor (EGF) (Sigma Chemical, catalog number: E9644 )
- 100x Insulin Transferrin Selenium (ITS) (Life Technologies, catalog number: 41400-045 )
- Rosewell Park Memorial Institute (RPMI) Medium -1640 (ATCC, catalog number: 41400-045)
- 50 μM Hydrocortisone (Sigma-Aldrich, catalog number: H6909 )
- Boveine serum albumin (BSA) (US Biochem, catalog number: 10857 )
- Disposable sterile tissue culture filters (Corning, catalog number: 431161 )
- Choline/acetylcholine Quantification Kit (BioVision, catalog number: K615-100 )
- Liquid nitrogen
- Serum-Free RPMI (SF-RPMI) (see Recipes)
- Neostigmine (Sigma Chemical, catalog number: N2001 ) (see Recipes)
Equipment
- 60 mm cell culture dishes (Corning, catalog number: 353002 )
- Microfuge tube
- ELISA Reader
- Lyophilizer (Labonco)
- Centrifuge
- CO2 cell culture incubator
- -80 °C freezer
Procedure
- A549 cells were grown in 5ml serum-free RPMI (SF-RPMI) to 90%-95% confluence in 60 mm cell culture dishes in a cell culture incubator set at 5% CO2 and 37 °C (Lau et al., 2013; Song et al., 2008; Song et al., 2003).
- On the day of the assay, 100 μM neostigmine (a chemical inhibitor of AChE in cells) was added to each plate for four hours at 37 °C. The plate contained 3 ml of media. Please see Notes section about optimization of the concentration of neostigmine.
- Four hours after the addition of neostigmine, the relevant concentration of test compund (which promotes/inhibits the secretion of ACh) was added and the cells were incubated at 37 °C for 36 h. An example of a compound which promotes the production of ACh is 100 nM nicotine.
- The supernatant (medium) was collected and spun at 800 x g.
- The supernatants were frozen at -80 °C and then lyophilized. The lyophilizer was set to a pressure of 10 micron Hg or below that value. The samples were lyophilized overnight.
- Subsequently, the lyophilizate was reconstituted with 1/5 volume autoclaved water (600 μl autoclaved water), snap frozen in liquid nitrogen and stored at -80 °C until further analysis.
- The amount of ACh in the sample was measured using the Choline/acetylcholine Quantification Kit, according to manufacturer’s instructions (http://www.biovision.com/choline-acetylcholine-quantification-colorimetric-fluorometric-kit-2910.html). The protocol outlined in the assay kit will be attached here. Each sample was assayed in triplicate.
Notes
ACh is secreted by lung cancer cells into the extracellular environment. Part of the ACh binds to nicotinic acetylcholine receptors and muscarinic acetylcholine receptors on the same lung cancer cells stimulating their proliferation in an autocrine manner. The excess ACh is quickly degraded by the enzyme AChE to generate choline which is then taken up by the cells to synthesize new ACh. Therefore, it is essential to inhibit the AChE to measure the ACh produced by the lung cancer cells.
Optimization of the concentration of neostigmine:
- Neostigmine is added to inhibit the enzyme AChE in cells. It is important to titrate the amount of neostigmine to be used otherwise it may compromise the readout of the assay.
- A549 cells were grown in serum-free RPMI (SF-RPMI) to 90%-95% confluence in 60 mm cell culture dishes in a cell culture incubator set at 5% CO2 and 37 °C.
- On the day of the assay, the following concentrations of neostigmine were added
- 10 μM
- 20 μM
- 50 μM
- 100 μM
- 200 μM
- 300 μM
- 10 μM
- Each plate contained 3 ml of media. The cells were incubated at 37 °C for 36 h.
- Follow the steps 4-7 of the Procedure section described above.
- In our experiments, the production of ACh varied with increasing concentrations of neostigmine as shown below in Figure 1A. Figure 1B shows that the amount of AChE in all three cell lines is similar. The baseline amount of ACh is highest in the presence of 100-200 uM neostigmine. Therefore, we selected 100 uM neostigmine for all our experiments. It is probable that 300 uM of neostigmine is toxic so the cells and causes cell death so the levels of ACh are lower.
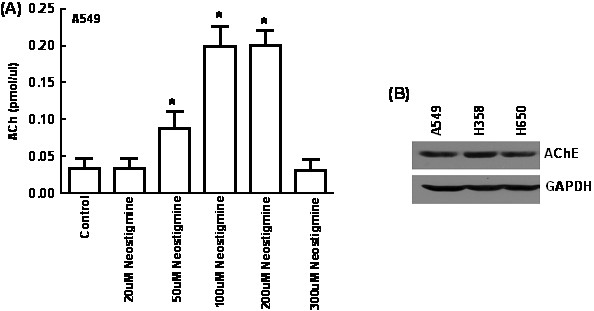
Figure 1. Optimization of the concentration of neostigmine for measurement of ACh from A549 human lung cancer cells. (A) A549 human lung cancer cells were cultured in SF-RPMI and the amount of ACh produced was measured in the presence of varying concentrations of neostigmine. The baseline amount of ACh is highest in the presence of 100-200 uM neostigmine. (B) Western blotting analysis shows the presence of robust amounts of AChE in three human lung cancer cell lines namely A549, H358 and H650. Values indicated by the * are statistically significant relative to controls (*P<0.05).
Recipes
- Serum-Free RPMI (SF-RPMI), 100 ml
To 50 ml of RPMI in a sterile bottle, add the following:
100 mg BSA to and stir to dissolve
1 ml ITS
100 ul of 50 uM hydrocortisone
1 mg EGF
Make up the volume to 100 ml with RPMI
Filter sterilize using a 0.22 μm filter
Stored at 4 °C
- Neostigmine (Stock = 100 mM)
Weigh 33.4 mg of neostigmine in a sterile microfuge tube
Dissolve it in autoclaved water
Aliquot in multiple microfuge tubes
Stored at -20 °C
Acknowledgments
We would like to acknowledge the following publications on which this protocol is based: Song et al. (2008); Song et al. (2003a); Song et al. (2003b). We thank Dr. Srikumar Chellappan and his laboratory for their continuous support. This work was supported by the grants Young Clinical Scientist Award (#82115) from the Flight Attendant Medical Association, Miami, FL and 1R15CA161491-01A1 from NIH to PDG. KCB is a recipient of a graduate fellowship from the WVSGC.
References
- Cancer, I.A.R.C. (2004). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Tobacco Smoke and Involuntary Smoking: IARC, Lyon, France. 51-1187.
- Lau, J. K., Brown, K. C., Thornhill, B. A., Crabtree, C. M., Dom, A. M., Witte, T. R., Hardman, W. E., McNees, C. A., Stover, C. A., Carpenter, A. B., Luo, H., Chen, Y. C., Shiflett, B. S. and Dasgupta, P. (2013). Inhibition of cholinergic signaling causes apoptosis in human bronchioalveolar carcinoma. Cancer Res 73(4): 1328-1339.
- Song, P., Sekhon, H. S., Fu, X. W., Maier, M., Jia, Y., Duan, J., Proskosil, B. J., Gravett, C., Lindstrom, J., Mark, G. P., Saha, S. and Spindel, E. R. (2008). Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res 68(12): 4693-4700.
- Song, P., Sekhon, H. S., Jia, Y., Keller, J. A., Blusztajn, J. K., Mark, G. P. and Spindel, E. R. (2003). Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res 63(1): 214-221.
- Song, P., Sekhon, H. S., Proskocil, B., Blusztajn, J. K., Mark, G. P. and Spindel, E. R. (2003). Synthesis of acetylcholine by lung cancer. Life Sci 72(18-19): 2159-2168.
- The HEALTH Consequences of Smoking. (2004). A report of the Surgeon General's Office on Smoking and Health, DHHS, Washington DC.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lau, J. K., Brown, K. C. and Dasgupta, P. (2013). Measurement of Acetylcholine from Cell Lines. Bio-protocol 3(24): e1007. DOI: 10.21769/BioProtoc.1007.
Category
Cancer Biology > Cellular energetics > Cell biology assays > Metabolism
Cell Biology > Cell metabolism > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link