zapERtrap: A light-regulated ER release system to study secretory protein trafficking
Speaker: Ashley M. Bourke
Online live: Nov 19, 2024 11:00 AM EST Posted: Dec 11, 2024 Views: 8591
Abstract
The secretory network, which includes the endoplasmic reticulum (ER), the ER-Golgi intermediate compartment (ERGIC), and the Golgi apparatus, orchestrates the synthesis, processing, and delivery of integral membranes and secreted proteins to their specific cellular destinations. Traditional methods for investigating these complex pathways have provided foundational insights but often lack the precision to control and observe protein trafficking at single-cell or subcellular levels.
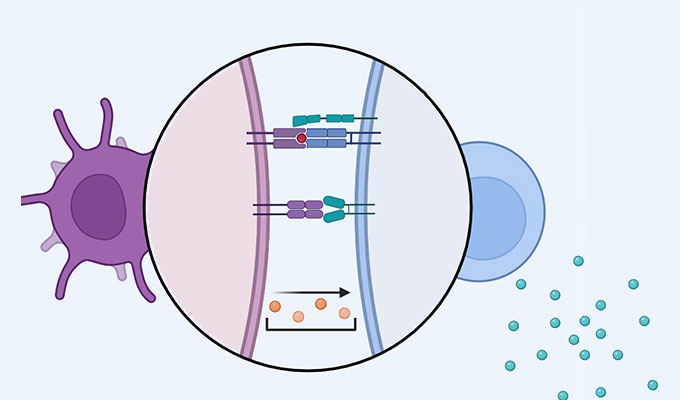
Zapalog-mediated endoplasmic reticulum trap (zapERtrap) addresses this gap by employing a brief pulse of visible light to trigger the release of diverse cargo proteins from the ER, offering exceptional spatial and temporal control. This method allows researchers to study protein trafficking with single-cell and subcellular resolution. This innovative approach builds on previous "trap and release" strategies but surpasses them by eliminating the need for bulky protein tags and UVB light, which posed limitations on local activation.
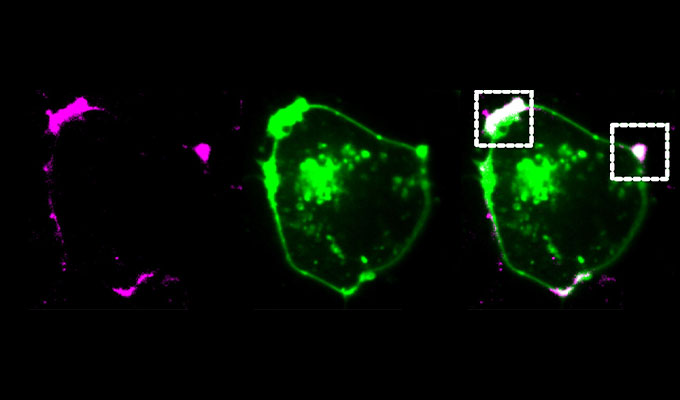
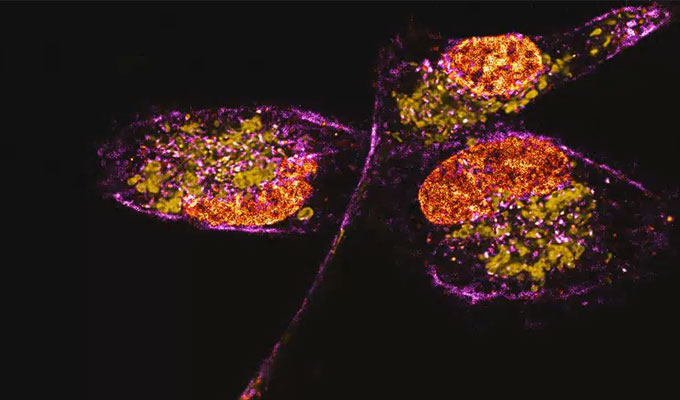
The zapERtrap system can be applied to various cellular processes, including the regulation of receptor levels, growth factors, morphogens, and hormones. This tool is especially useful in neurons, enabling the study of protein regulation across dendritic and axonal projections. ZapERtrap allows researchers to track where synaptic proteins, like neuroligin 1 (NL1) and GluA1-containing AMPA receptors, appear on the cell surface. Overall, zapERtrap's ability to provide tunable and precise control over protein trafficking makes it an invaluable tool for advancing our understanding of cellular organization and function, with broad applications in cell biology and beyond.
In this webinar, we will discuss the development and functionality of zapERtrap, its proof-of-concept applications in neuroscience for studying the trafficking of synaptic proteins, and its potential for broader applications in cell biology. Attendees will gain insights into how zapERtrap can be used to dissect complex secretory pathways and reveal new aspects of cellular organization and function.
Highlights
- Overview of “trap and release” strategies for controlling secretory trafficking
- Introduction to zapERtrap: Understanding the principles behind this light-regulated ER release system and how it enhances spatial and temporal control of protein trafficking.
- zapERtrap applications in neuroscience and the study of compartmentalized trafficking.
- Technical advancements and broad applications of zapERtrap.
Speaker

Ashley M. Bourke, Ph.D.
Postdoctoral Fellow, Max Planck Institute for Brain Research
Dr. Ashley Bourke is a Postdoctoral Fellow in Dr. Erin Schuman's lab at the Max Planck Institute for Brain Research in Frankfurt, Germany. Dr. Ashl...
View more
Keywords
zapERtrap, Protein trafficking and secretion pathways, Light-regulated release for high spatial and temporal control, Subcellular resolution, Neuroscience
References
Ashley M. Bourke, Samantha L. Schwartz, Aaron B. Bowen, Mason S. Kleinjan, Christina S. Winborn, Dean J. Kareemo, Amos Gutnick, Thomas L. Schwarz, Matthew J. Kennedy; zapERtrap: A light-regulated ER release system reveals unexpected neuronal trafficking pathways. J Cell Biol 6 September 2021; 220 (9): e202103186. doi: https://doi.org/10.1083/jcb.202103186
Bourke AM, Kennedy MJ. Spatial and Temporal Control of Protein Secretion with Light. Methods Mol Biol. 2022;2473:29-45. doi:10.1007/978-1-0716-2209-4_4
Do you have a question about this webinar?
Post your question, and we'll invite the webinar speaker to respond. You're welcome to join the discussion by answering or commenting on questions ( Note: Not all questions, especially those not directly relevant to the webinar topic, may be answered by the speaker. ).
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question.
9 Q&A