- EN - English
- CN - 中文
Bimolecular Fluorescence Complementation (BiFC) Assay for Direct Visualization of Protein-Protein Interaction in vivo
通过双分子荧光互补(BiFC)试验进行体内蛋白质之间的相互作用的可视化分析
发布: 2013年10月20日第3卷第20期 DOI: 10.21769/BioProtoc.935 浏览次数: 24661
Abstract
Bimolecular Fluorescence Complementation (BiFC) assay is a method used to directly visualize protein-protein interaction in vivo using live-cell imaging or fixed cells. This protocol described here is based on our recent paper describing the functional association of human chromatin adaptor and transcription cofactor Brd4 with p53 tumor suppressor protein (Wu et al., 2013). BiFC was first described by Hu et al. (2002) using two non-fluorescent protein fragments of enhanced yellow fluorescent protein (EYFP), which is an Aequorea victoria GFP variant protein, fused respectively to a Rel family protein and a bZIP family transcription factor to investigate interactions between these two family members in living cells. The YFP was later improved by introducing mutations to reduce its sensitivity to pH and chloride ions, thus generating a super-enhanced YFP, named Venus fluorescent protein, without showing diminished fluorescence at 37 °C as typically observed with EYFP (Nagai et al., 2006). The fluorescence signal is regenerated by complementation of two non-fluorescent fragments (e.g., the Venus N-terminal 1-158 amino acid residues, called Venus-N, and its C-terminal 159-239 amino acid residues, named Venus-C; see Figure 1A and Gully et al., 2012; Ding et al., 2006; Kerppola, 2006) that are brought together by interaction between their respective fusion partners (e.g., Venus-N to p53, and Venus-C to the PDID domain of human Brd4; see Figure 1B and 1C). The intensity and cellular location of the regenerated fluorescence signals can be detected by fluorescence microscope. The advantages of the proximity-based BiFC assay are: first, it allows a direct visualization of spatial and temporal interaction between two partner proteins in vivo; second, the fluorescence signal provides a sensitive readout for detecting protein-protein interaction even at a low expression level comparable to that of the endogenous proteins; third, the intensity of the fluorescence signal is proportional to the strength of protein-protein interaction (Morell et al., 2008); and fourth, the BiFC signals are derived from intrinsic protein-protein interaction, rather than from extrinsic fluorophores that may not reflect true protein-protein interaction due to their nonspecific association with cellular macromolecules or subcellular compartments. However, some limitations of BiFC include slow maturation (T1/2 ~ 1 hour) of an eventually stable BiFC complex (Hu et al., 2002), making it unsuitable for real-time observation of transient interaction that disappears prior to BiFC detection, and enhanced BiFC background at high expression levels due to fusion-independent association between two non-fluorescent fragments association. BiFC signals generated by in vivo protein-protein interaction can be validated by amino acid mutation introduced at the protein-protein contact surfaces. This imaging technique has been widely used in different cell types and organisms (Kerppola, 2006).
Keywords: BiFC (互补试验)

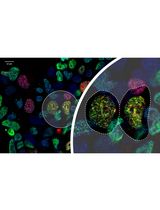
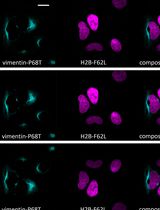
Figure 1. Protein fragments of Venus (super enhanced YFP) constructs. A. Venus protein (amino acids 1-239; accession number: CAO79509) was dissected into two fragments at residue 158 to generate Venus N-terminus (top) and Venus C-terminus (bottom). B. Schematic drawing of Venus-N-p53 and Venus-C-PDID fusion fragments. Venus-N-p53 and Venus-C-PDID contain Venus-N-terminus and Venus-C-terminus fused respectively to p53 (amino acids 1-393; Gully et al., 2012) and the phosphorylation-dependent interaction domain (PDID, amino acids 287-530) of human Brd4 (Wu et al., 2013), in which a flexible linker containing two copies of Gly4Ser peptide is introduced to allow optimal space contacts between Venus-N-terminus and Venus-C-terminus and also to prevent steric hindrance between the Venus fragment and its fused protein of interest. AscI and XbaI indicate the positions of restriction enzyme-cutting sites used for generating fusions from PCR-amplified DNA fragments. An initiation codon for methionine (M) was added to allow translation of Venus-C-PDID. It should be noted that, although linker peptides ranging from 5 to 17 amino acids are often used (Remy and Michnick, 2007), the exact length and the sequence nature of the linkers have not been systematically analyzed (Kerppola, 2013). C. BiFC fluorescence signal is produced when Venus-N and Venus-C are in close proximity brought together via p53-PDID interaction in the cell.
Materials and Reagents
- Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F2442 )
- Antibiotics (Penicillin/Streptomycin) (Sigma-Aldrich, catalog number: P0781 )
- Cell culture medium (Complete: With 10% FBS and antibiotics; Antibiotic-free: with 10% FBS only)
- Formaldehyde (Thermo Fisher Scientific, catalog number: F79-500 )
- Triton X-100 (Sigma-Aldrich, catalog number: 79284 )
- BSA (Sigma-Aldrich, catalog number: A3059 )
- Lipofectamine 2000 (Life Technologies, Invitrogen™, catalog number: 11668-019 )
- Venus-N-p53 (Gully et al., 2012) (Santa Cruz, catalog number: sc8334 ) and Venus-C-PDID (Wu et al., 2013) plasmids (Santa Cruz, catalog number: sc5384 ) (see Figure 1B)
- Primary antibodies against Venus
e.g. Anti-full-length-GFP antibody (Santa Cruz, catalog number: sc8334 or sc9996 )
Anti-C-terminal GFP antibody (Santa Cruz, catalog number: sc5384)
or β-actin (Sigma-Aldrich, catalog number: A5441 )
- Secondary antibody conjugated with a fluorescence dye emitting wavelength other than that of Venus (excitation 488 nm, emission 515 ± 15 nm) or Hoechst 33258 (excitation 350 nm, emission 461 nm), for example, Alexa Fluor® from Life Technologies
- Sodium chloride (Thermo Fisher Scientific, catalog number: 7647-14-5 )
- Potassium chloride (Thermo Fisher Scientific, catalog number: 7447-40-7 )
- Sodium phosphate dibasic heptahydrate (Na2HPO4.7H2O) (Thermo Fisher Scientific, catalog number: S373500 )
- Potassium phosphate monobasic (KH2PO4) (Thermo Fisher Scientific, catalog number: 7778-77-0 )
- Aluminum foil (grocery store)
- Permanent mounting medium (Vector Laboratories, catalog number: H-5000 )
- Microscope slides (Thermo Fisher Scientific, catalog number: 12-544-7 )
- Nail polish (grocery store)
- 10x Phosphate Buffered Saline (PBS) (see Recipes)
- 3.7% Formaldehyde (freshly prepared) (see Recipes)
- Phosphate Buffered Saline with Triton X-100 and BSA (PBSTB) (see Recipes)
- Hoechst 33258 (Sigma-Aldrich, catalog number: 861405 ) (see Recipes)
Equipment
- Glass bottom culture dish (35-mm glass bottom plate containing a 14-mm center microwell, poly-D-lysine coated) (MatTek, catalog number: P35GC-1.5-14-C )
- Tissue culture hood (NuAire, model: Class II, Type A2 )
- 37 °C cell culture incubator (Thermo Fisher Scientific, Forma®, model: Series II , water-jacketed and HEPA filtered)
- Confocal fluorescence microscope (Nikon, model: Eclipse TE-2000E/C1 )
- Rocker (Labnet International, model: Rocker 25 )
Software
- NIS Elements Basic Research (version 2.2)
- Nikon EZ-C1 Free Viewer (version 3.90)
Procedure
文章信息
版权信息
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Lai, H. and Chiang, C. M. (2013). Bimolecular Fluorescence Complementation (BiFC) Assay for Direct Visualization of Protein-Protein Interaction in vivo. Bio-protocol 3(20): e935. DOI: 10.21769/BioProtoc.935.
分类
细胞生物学 > 细胞成像 > 荧光
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link