- EN - English
- CN - 中文
Determination of Enzyme Kinetic Parameters of UDP-glycosyltransferases
UDP糖基转移酶动力学常数的测定
发布: 2013年07月20日第3卷第14期 DOI: 10.21769/BioProtoc.825 浏览次数: 13078
评审: Tie Liu
Abstract
The determination of enzyme kinetic parameters, such as the Km and kcat values, is an essential part of the characterization of newly discovered enzymes. This protocol describes the determination of enzyme kinetic parameters of the Barbarea vulgaris UDP-glycosyltransferases (UGTs) UGT73C11 and UGT73C13 toward the sapogenins oleanolic acid and hederagenin as sugar acceptor substrates. UGTs catalyze the transfer of glycosyl residues. They generally use uridine sugar nucleotides as their sugar donor substrates, whereas sugar acceptor substrates arise from structurally diverse sets of metabolite classes. This protocol is based on the quantification of 14C-labeled glycosides following thin layer chromatography (TLC)-based separation. The dependence of the measured signal on a universal radioactively-labeled sugar donor substrate allows the potential application of the protocol in combination with a wide range of different sugar acceptor substrates. However, since the here described TLC separation procedure has been optimized for the separation of sapogenins and their glycosides, some modifications may become necessary when investigating other compound classes.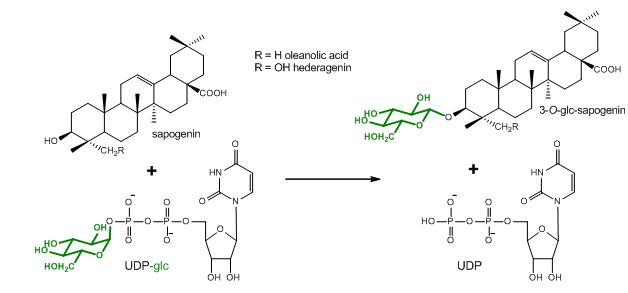
Figure 1. Glucosylation reaction catalyzed by UGT73C10-UGT73C13 from Barbarea vulgaris (Augustin et al., 2012). All four enzymes utilize uridine diphosphate glucose (UDP-glc) as glucosyl-moiety donor substrate and different sapogenins such as the oleanane sapogenins oleanolic acid and hederagenin as glucosyl-moiety acceptor substrates.
Materials and Reagents
- Uridine-5'-diphosphoglucose (UDP-Glc) (e.g. Sigma-Aldrich, catalog number: S451649 )
- Uridine-5'-diphosphate-[14C]glucose (UDP-[14C]Glc) (e.g. PerkinElmer, catalog number: NEC403050UC )
- N-Tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid (TAPS) (e.g. Sigma-Aldrich, catalog number: T5130 )
- Bovine serum albumin (BSA) (e.g. Sigma-Aldrich, catalog number: A7906 )
- Dithiothreitol (DTT) (e.g. Sigma-Aldrich, catalog number: D0632 )
- Silica gel 60 F254 plates (e.g. EMD Millipore, catalog number: 1055540001 )
- Appropriate E. coli protein expression strain (e.g. strain XJb(DE)) (Zymo Research, catalog number: T5051 )
- FRETWorks S-tag assay kit (EMD Millipore, catalog number: 70724 )
- TAPS buffer
- 1.6 mM oleanolic acid stock solution (see Recipes)
- 1.6 mM hederagenin stock solution (see Recipes)
- Pre-master mix for 36 UGT73C11 enzyme assay reactions (see Recipes)
- Pre-master mix for 37 UGT73C13 enzyme assay reactions (see Recipes)
Equipment
- Vacuum centrifuge (e.g. Labogene, catalog number: 7.008.100.777 )
- TLC Developing Chamber (e.g. VWR international, catalog number: 21432-739 )
- Storage phosphor screens (e.g. GE healthcare, catalog number: 28-9564-74 )
- Phosphorimager (e.g. Molecular Dynamics, model: STORM 840 )
Software
- ImageQuant 5.0 (Molecular Dynamics) or similar
- SigmaPlot 11.0 (Systat Software, Inc.) or similar
Procedure
文章信息
版权信息
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Augustin, J. M. and Bak, S. (2013). Determination of Enzyme Kinetic Parameters of UDP-glycosyltransferases. Bio-protocol 3(14): e825. DOI: 10.21769/BioProtoc.825.
- Augustin, J. M., Drok, S., Shinoda, T., Sanmiya, K., Nielsen, J. K., Khakimov, B., Olsen, C. E., Hansen, E. H., Kuzina, V., Ekstrom, C. T., Hauser, T. and Bak, S. (2012). UDP-glycosyltransferases from the UGT73C subfamily in Barbarea vulgaris catalyze sapogenin 3-O-glucosylation in saponin-mediated insect resistance. Plant Physiol 160(4): 1881-1895.
分类
植物科学 > 植物生物化学 > 蛋白质 > 活性
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link