- EN - English
- CN - 中文
Electroporation of Whole-Mount Postnatal Rodent Retinas for Advanced Functional Assays
发布: 2026年01月20日第16卷第2期 DOI: 10.21769/BioProtoc.5574 浏览次数: 27
评审: Anonymous reviewer(s)
Abstract
To study gene function in regulating rodent retinal waves during development, an efficient method for gene delivery into whole-mount retinas is required while preserving circuit functionality for physiological studies. We present an optimized electroporation protocol for developing rodent retinal explants. The procedure includes the fabrication of horizontally aligned platinum electrodes and the placement of retinal explants between them to generate a uniform electric field for high transfection efficiency. The entire process—dissection and electroporation—can be completed within 1–2 h. Successful transfection is verified by fluorescence microscopy, and physiological assays such as patch-clamp recordings and live imaging can be performed within 1–4 days following electroporation. This rapid and reliable protocol enables functional analysis for a specific gene in regulating retinal waves and can be adapted to other organotypic slice cultures.
Key features
• Incorporates horizontally aligned platinum electrodes and enables cell type–specific promoters to drive gene expression for physiological studies.
• Preserves retinal wave activity while markedly improving transfection efficiency in whole-mount postnatal rodent retinas.
• Requires only 1–2 h from retinal dissection to electroporation.
• Allows completion of functional experiments within four days after electroporation.
Keywords: ElectroporationGraphical overview
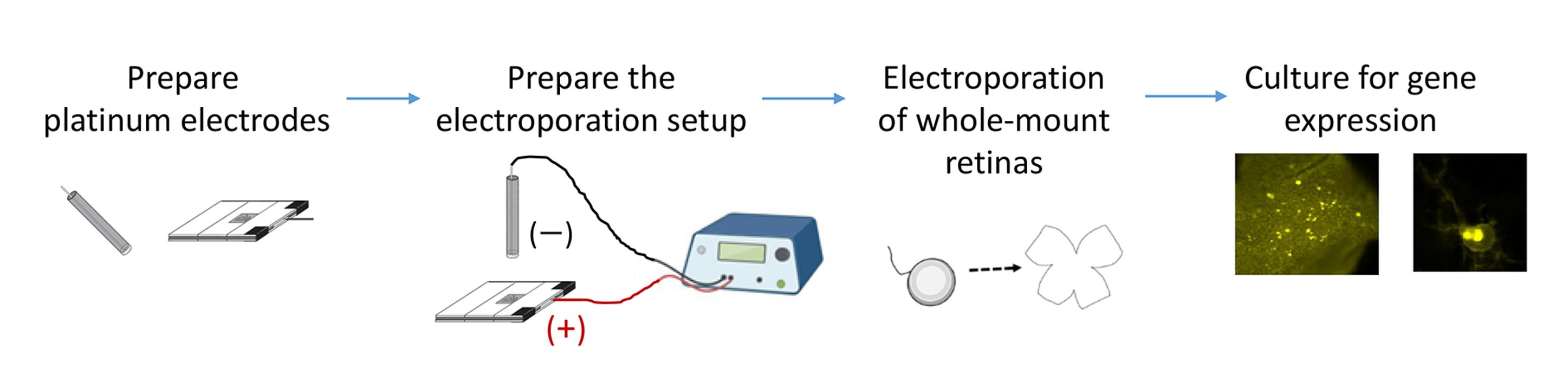
Background
A hallmark of developing neural circuits is recurrent, patterned spontaneous activity observed in many regions of the central nervous system, such as retinal waves in the developing visual system [1–4]. To investigate the molecular basis of this circuit activity, it is essential to combine gene delivery with physiological assays in the developing whole-mount retina. Among existing gene delivery methods—including transgenic approaches, viral infection, and biolistic delivery—electroporation provides a rapid, efficient, and flexible alternative. Unlike viral vectors, electroporation can express ectopic genes that may otherwise recombine with similar sequences, such as CFP and YFP, which interfere with FRET-based genetically encoded reporters [5]. These reporters, such as kinase activity sensors, have been successfully introduced into cells by microinjection [6], lipofection [7], or electroporation [8]. Both electroporation and biolistic transfection rely on physical means of macromolecule entry—via transient pore formation or membrane penetration, respectively—but electroporation is generally more cost-effective and efficient for postmitotic neurons. A key challenge in whole-mount retina electroporation is maintaining circuit functionality and preserving retinal waves.
Electroporation has been adapted for various experimental purposes, but each method has limitations. Commercial electroporation cuvettes with aluminum electrodes are widely used for dissociated neurons; however, they can generate toxic free radicals [9] and provide limited control over tissue orientation, critical for studies such as retinotopic mapping. Single-cell electroporation is effective for analyzing individual neurons but achieves limited transfection coverage, insufficient for manipulating network-level activity [10–12]. In vivo and in utero electroporation techniques have been applied to adult, neonatal, and embryonic rodents [13–18], yet they require extensive surgical skill and animal handling. Thus, a more accessible and scalable approach is needed to combine efficient gene delivery with preserved physiological properties in the developing whole-mount retina.
To address this need, we developed an optimized electroporation protocol for whole-mount rodent retinas that achieves high transfection efficiency while maintaining native retinal circuit function. The protocol described here is based on previous works [8,19–24]. We provide detailed descriptions of the construction and use of horizontally aligned platinum electrodes that generate a uniform electric field across the developing retina. Whole-mount retinal explants are positioned between the electrodes, and DNA solution is introduced into the gap to facilitate efficient and homogeneous gene transfer. These horizontally aligned platinum electrodes avoid the potential toxicity of aluminum electrodes and provide better control over tissue orientation. As a result, retinal waves remain intact following transfection, ensuring compatibility with physiological studies, including patch-clamp recordings and live imaging. This electroporation method has been successfully used to introduce the A-kinase activity reporter (AKAR) into retinal explants, enabling real-time monitoring of the temporal relationship between PKA activation and retinal waves—the characteristic spontaneous activity of developing retinas [8,19]. The protocol has also been adapted for cell type–specific gene expression, for example, using the mGluR2 promoter to target type II metabotropic glutamate receptor–expressing neurons [20–24], highlighting the versatility of the approach in dissecting the signaling pathways and cell-specific contributions that shape neural circuit development. This method requires only 1–2 h from retinal dissection to electroporation and preserves circuit integrity for up to several days in culture. Functional experiments, including patch-clamp recordings and live imaging, can be performed within four days after transfection. Researchers using this protocol are expected to be familiar with basic physiological techniques, which are described elsewhere [25–27]. Together, this optimized electroporation protocol enables short-term functional studies of gene regulation in neural circuit development. The same principles can be applied to other organotypic slice cultures, offering a versatile platform for investigating molecular and cellular mechanisms underlying neural circuit assembly.
Materials and reagents
Biological materials
1. Experimental animals: Postnatal Sprague–Dawley rats (P0-P2) or C57BL/6J mice (P0-P2)
Reagents
1. Hanks’ balanced salt solution (HBSS) (10×), calcium, magnesium, no phenol red (Gibco, catalog number: 14065056)
2. HEPES (Sigma, catalog number: H7523)
3. NaHCO3 (Sigma, catalog number: S-6014)
4. QIAGEN Plasmid Mega kit (Qiagen, catalog number: 12181, 12183, or 12281)
5. Tris (Sigma, catalog number: T6066; dissolve the powder in ddH2O to prepare a pH 7.4 solution)
6. Neurobasal-A medium (Gibco, catalog number: 10888022)
7. Glucose (Sigma, catalog number: G7528)
8. L-glutamine (Sigma, catalog number: G-6392)
9. B-27 supplement (50×), serum-free (Gibco, catalog number: 17504-044)
10. Sodium pyruvate (100 mM) (Gibco, catalog number: 11360070)
11. Insulin from bovine pancreas (Sigma, catalog number: I-1882-100MG)
12. Bovine serum albumin (BSA) (Cyrusbioscience, catalog number: 101-9048-46-8)
13. HCl (Sigma, catalog number: H1758)
14. Penicillin-Streptomycin (10,000 U/mL) (Gibco, catalog number: 15140-122)
15. Ciliary neurotrophic factor (CNTF) (100 μg/mL stock) (PeproTech, catalog number: 450-13)
16. Brain-derived neurotrophic factor (BDNF) (500 μg/mL stock) (PeproTech, catalog number: 450-02)
17. Forskolin (Sigma, catalog number: F-6886)
18. Dimethyl sulfoxide (DMSO) (sterile) (Sigma, catalog number: E-2438)
19. Ethanol (Sigma, catalog number: E-7023)
Solutions
1. Retinal dissection buffer (see Recipes)
2. DNA plasmid-containing solution (see Recipes)
3. Retinal serum-free culture medium–adult (SFCM-A) (see Recipes)
4. SFCM-A with forskolin (see Recipes)
Recipes
1. Retinal dissection buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HBSS (10×) | 1× | 100 mL |
| HEPES (1 M stock with pH 7.35–7.4) | 10 mM | 100 mL |
| NaHCO3 | 0.035% (w/v) | 0.35 g |
| ddH2O | n/a | n/a |
| Total | n/a | 1 L |
Critical: Mix all ingredients well and adjust the pH to 7.35. Filter-sterilize by vacuum filtration. Aliquot 50 mL per tube to avoid contamination. Store sterile at -20 °C.
2. DNA plasmid-containing solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DNA plasmid stock (2 μg/μL) purified with the QIAGEN Plasmid Mega kit; dissolved in 10 mM Tris buffer, pH 7.4 | 0.2 μg/μL | 40 μL |
| Retinal dissection buffer | n/a | 360 μL |
| Total | n/a | 400 μL |
Critical: Store DNA plasmid stock at -20 °C. Aliquot the DNA plasmid-containing solution in 200 μL per Eppendorf to avoid the freeze-thaw-induced DNA fragmentation. For electroporation, freshly prepare sufficient DNA plasmid-containing solution for all explants using the following calculation: 400 μL × (the number of explants + 1). Use all DNA plasmid-containing solution; do not store leftovers, as DNA degrades readily in the dissection buffer.
3. Retinal serum-free culture medium–adult (SFCM-A)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Neurobasal-A medium | n/a | 47 mL |
| Glucose | 0.6% w/v | 0.3 g |
| L-glutamine (200 mM stock in ddH2O) | 2 mM | 500 μL |
| B-27 supplement (50×) | 1× | 1 mL |
| HEPES (1 M stock, pH 7.35–7.4) | 10 mM | 500 μL |
| Sodium pyruvate (100 mM) | 1 mM | 500 μL |
| Insulin (200× stock) | 2.5 μg/mL | 250 μL |
| Penicillin-streptomycin (10,000 U/mL) | 50 U/mL | 250 μL |
| CNTF (100 μg/mL stock) | 10 ng/mL | 5 μL |
| BDNF (500 μg/mL stock) | 50 ng/mL | 5 μL |
| Total | n/a | 50 mL |
Critical: Mix all well. Filter-sterilize by vacuum filtration. Prepare fresh. Store at -20 °C for 1 month or at 4 °C for ready use within 2 days.
Critical: For postnatal tissue culture, we must purchase Neurobasal-A (“A” stands for adult) from Gibco (catalog numbers 10888 with phenol red and 12349 without phenol red), which has an optimal osmolarity for postnatal and adult neurons. In contrast, Neurobasal from Gibco (catalog numbers 21103 with phenol red and 12348 without phenol red) has a relatively low osmolarity, which is suitable for embryonic neural tissue.
Critical: The recipe for 200× insulin stock is shown below; it contains 0.5 mg/mL insulin in 3 mM HCl and 0.1% BSA (pH 2.5). In the presence of 3 mM HCl, the pH is approximately 2.5. 0.1% BSA is required to stabilize insulin. Filter-sterilize, aliquot, and store at -20 °C.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Insulin | 0.5 mg/mL | 10 mg |
| BSA | 0.1% w/v | 20 mg |
| HCl (6 N) | 3 mM | 10 μL |
| ddH2O | n/a | bring the final volume to 20 mL |
4. SFCM-A with forskolin
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Forskolin (30 mM stock) | 6 μM | 5 μL |
| SFCM-A | n/a | 25 mL |
Critical: Prepare fresh in the sterile hood. Store at -20 °C for 1 month or at 4 °C for ready use within 2 days. Aliquot the stocks in 20 μL per Eppendorf to reduce the freeze-thaw cycles to four times. Keep sterile at -20 °C.
Laboratory supplies
1. Platinum foil, 25 × 25 mm, 99.99% (Aldrich, catalog number: 349364–350 mg)
2. Ball-point pen (Tempo, catalog number G-102; also compatible with transparent ball-point pens with an outer barrel diameter of ~1 cm, an inner diameter >0.6 cm, and a body length >11.5 cm)
3. Electric wires, multi-core tinned copper, O.D. 1 mm with insulation, in red and black for the positive and negative electrodes, respectively (TopTech Wire and Cable, catalog number: 1007#24)
4. Electrical insulation tape (3M, catalog number: B40070579)
5. Epoxy glue (AB glue) (Success, catalog number: 1632), aluminum foil (Kirkland Signature, catalog number: RK611), and toothpicks (Diamond SKU, catalog number: 535-376-821)
6. Microscope slides, 8 slides (size “1 × 3”; thickness 1–1.2 mm) (Paul Marienfeld, catalog number: 1000412)
7. Soldering iron (Taiyo Electric Ind. Co., Ltd., catalog number: TQ-95)
8. Soldering tin (Shenmao Technology Inc., catalog number: PF606-R): lead-free SAC alloy (Sn 96.5%, Ag 3.0%, Cu 0.5%) with a rosin-based, non-acid “no-clean” flux; the recommended working temperature is typically 280–350 °C
9. BNC cable (Cal Test Electronics, catalog number: CT4452-100): one end features two banana plugs for connection to the electroporator, and the other end has a connector for attaching to the wires from the positive and negative electrodes
10. Stainless steel plate (e.g., a divider from an office cabinet) (UB office furniture, catalog number: UB4-30P)
11. Circular bubble level (Dogger, catalog number: D99C-09003)
12. Petri dish, 3.5 cm (Corning, catalog number: 430165)
13. Kimwipes (Kimtech, catalog number: 34155)
14. Razor blade (GEM, catalog number: 62-0165)
15. Mini-dissecting scissors (World Precision Instruments, catalog number: 503246)
16. Filter paper, No. 1 (Whatman, catalog number: 1001090)
17. Scalpel handle, No. 3 (World Precision Instruments, catalog number: 500236)
18. Scalpel blades, No. 10 (World Precision Instruments, catalog number: 500239)
19. Scalpel blades, No. 11 (World Precision Instruments, catalog number: 500240)
20. Vannas scissors (World Precision Instruments, catalog number: 500086)
21. Petri dish, 10 cm (Corning, catalog number: 430167)
22. Paintbrush (Samyick, catalog number: No. 0 or No. 1) to handle and dissociate the retinas
23. Forceps, Tweezers Dumont No. 5 (World Precision Instruments, catalog number: 14095)
24. Glass droppers (Volac, catalog number: D812), fire-polished tips
25. Black nitrocellulose filter paper, 0.45 μm (Millipore, catalog number: HABP02500)
26. 24-well cell culture plate (Corning, catalog number: 3524)
27. Vacuum filtration devices (Nalgene 90-mm filter units for 500 mL, catalog number: 8-0000-230301)
28. Syringe filters (Pall Corp., catalog number: PN4612)
Equipment
1. Square-wave pulse electroporator (Harvard apparatus, catalog number: BTX ECM830)
2. Micromanipulator (Marzhauser, catalog number: MM33)
3. Magnetic stand (Marzhauser, catalog number: M-10)
4. Clean bench
5. Ice box
6. Dissection microscope (Leica, catalog number: EZ4D)
7. Cell culture hood
8. Humidified CO2 incubator, 5% CO2, 35 °C (Sanyo, catalog number: MCO-5AC)
Procedure
文章信息
稿件历史记录
提交日期: Nov 3, 2025
接收日期: Dec 17, 2025
在线发布日期: Dec 26, 2025
出版日期: Jan 20, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Huang, C., Chen, T., Su, Y., Tseng, C., Chen, P. and Wang, C. (2026). Electroporation of Whole-Mount Postnatal Rodent Retinas for Advanced Functional Assays. Bio-protocol 16(2): e5574. DOI: 10.21769/BioProtoc.5574.
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









