- EN - English
- CN - 中文
Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
发布: 2026年01月20日第16卷第2期 DOI: 10.21769/BioProtoc.5571 浏览次数: 21
评审: Samantha HallerAnonymous reviewer(s)
Abstract
Adipogenic differentiation efficiency remains highly variable across laboratories and cellular models, underscoring a critical need for a robust and standardized protocol. Here, we describe an optimized and highly effective protocol for inducing adipogenesis in multiple models, including murine 3T3-L1 preadipocytes, stromal vascular fraction (SVF) from neonatal and adult mice, and human adipose-derived stem cells (hADSCs). Systematic optimization was performed on key parameters such as initial cell confluence, induction timing, inducer composition, and culture surface coating. We show that high cell density, rosiglitazone supplementation, and an extended primary induction phase combine to promote lipid accumulation. Notably, we introduce a crucial modification—prolonged low-dose insulin stimulation during the maintenance phase—that is essential for the efficient differentiation of adult SVF. Furthermore, when applied to hADSCs, the protocol consistently induced robust adipogenesis, confirming its cross-species applicability. Taken together, this comprehensive and reproducible protocol serves as a valuable tool for advancing in vitro adipogenesis research.
Key features
• Extend a robust, standardized adipogenic differentiation protocol from 3T3-L1 preadipocytes to clinically relevant models, including hADSCs and the heterogeneous SVF.
• Identify key optimized parameters—cell density, induction timing, and inducer composition—enabling highly reproducible differentiation across species.
Keywords: Adipogenic differentiationGraphical overview
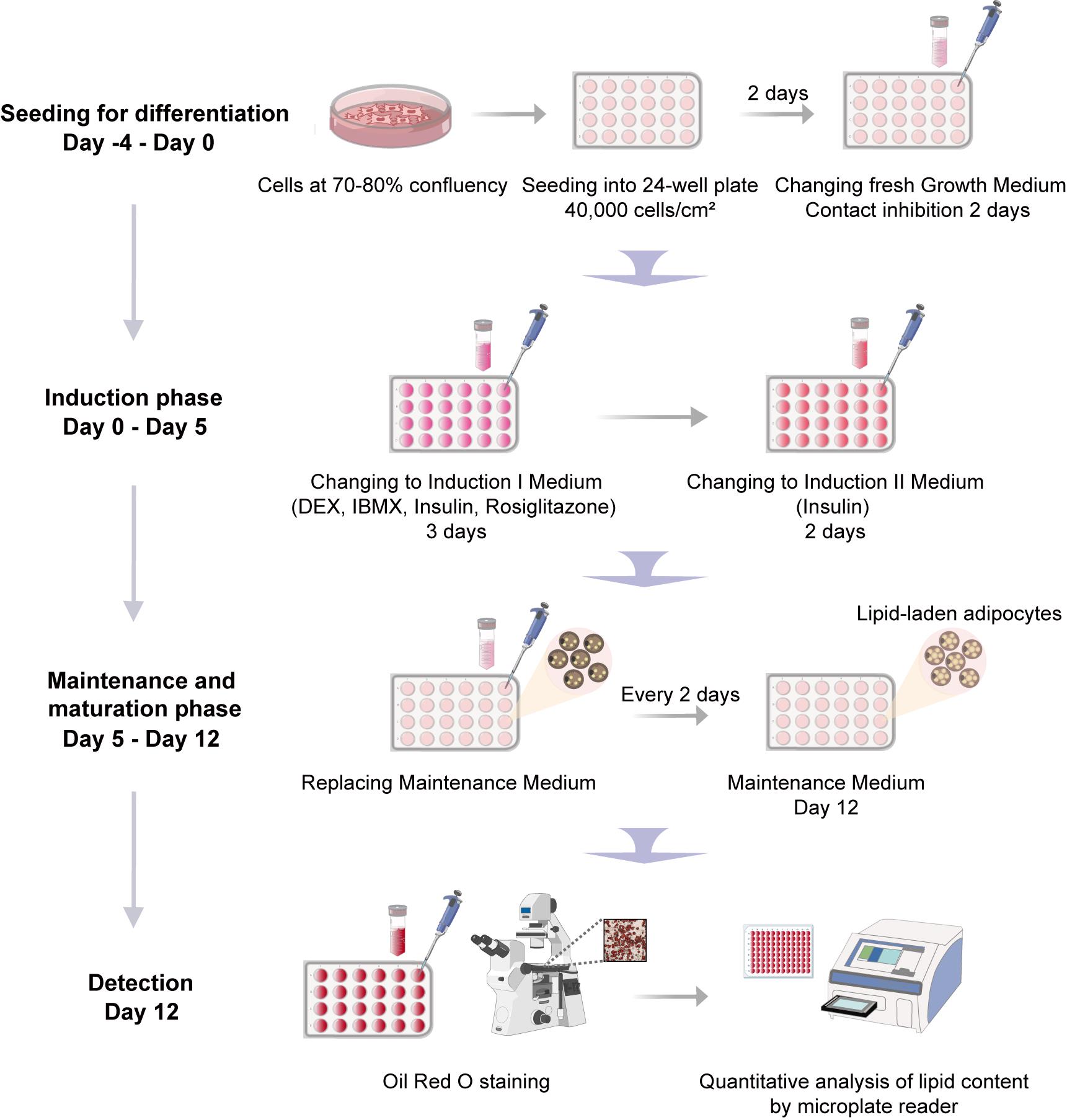
Schematic overview of the adipogenic differentiation protocol
Background
Adipogenic differentiation is a critical biological process with profound implications for metabolic health and regenerative medicine [1–3]. The escalating global burden of obesity and associated metabolic syndromes underscores the urgent need to elucidate the molecular mechanisms underlying adipocyte development and function [4]. However, progress in this field has been hampered by inconsistent differentiation outcomes, largely attributable to variations in cell sources, culture conditions, and induction protocols. These inconsistencies significantly compromise the reproducibility and translational relevance of adipogenesis research, highlighting the pressing demand for a standardized, efficient, and universally applicable differentiation methodology.
Adipogenesis, the process through which multipotent progenitor cells—including those of mesenchymal stromal and neural crest origin [5,6]—commit to preadipocytes and terminally differentiate into mature, lipid-laden adipocytes, is orchestrated by a well-defined transcriptional cascade [7,8]. Adipose-derived stem cells (ASCs) serve as a major cellular source of committed preadipocytes in adipose tissue [3]. This transformation is primarily governed by the master regulators peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein (C/EBP) family members [9]. The conventional adipogenic induction cocktail—composed of dexamethasone (DEX), 3-isobutyl-1-methylxanthine (IBMX), and insulin—has been widely employed as the standard methodology across cellular models [10,11]. Building upon this foundation, substantial efforts have been dedicated to protocol refinement. The recognition of PPARγ as the central regulator of adipogenesis prompted the incorporation of potent agonists such as rosiglitazone. As a thiazolidinedione-class antidiabetic drug, rosiglitazone improves glycemic control via insulin sensitization, while its ability to stimulate adipocyte differentiation and lipid accumulation accounts for the weight gain observed clinically [12,13]. This dual activity enables rosiglitazone to significantly enhance differentiation efficiency in both cell lines and primary preadipocytes [14,15]. Further strategies include prolonged IBMX exposure and the use of alternative PPARγ agonists like mifepristone [16,17]. Remarkably, single-agent mifepristone treatment is capable of inducing adipogenesis in 3T3-L1 preadipocytes [18].
Despite these advances, adipogenesis research continues to face substantial challenges in protocol standardization. Considerable variability exists in inducer concentrations across laboratories, with studies reporting different optimal levels of DEX, IBMX, and insulin, particularly when working with out-of-thaw or high-passage cells [17,19]. Additionally, differences in induction timing, serum lots, and culture conditions further contribute to inconsistent differentiation outcomes across cell sources and species [20,21]. The absence of a standardized, universally applicable protocol remains a major limitation in the field, hindering both reproducibility and translational applications.
In response to these challenges, we present a comprehensively optimized adipogenic differentiation protocol developed through systematic parameter optimization. Our method builds upon established approaches while introducing critical refinements to achieve superior performance across multiple cellular models, including 3T3-L1 preadipocytes, primary stromal vascular fraction (SVF) from neonatal and adult mice, and human adipose-derived stem cells (hADSCs). It should be noted that the inherent heterogeneity of SVF, which contains a mixture of fibroblasts, leukocytes, endothelial cells, and stromal cells, may contribute to variations in differentiation efficiency between replicates, donors, and laboratories. Nevertheless, this protocol offers a valuable standardized tool for investigating adipogenesis in diverse experimental settings.
Materials and reagents
Biological materials
1. 3T3-L1 preadipocytes (Cell Resource Center, Peking Union Medical College; NSTI-BMCR, catalog number: 1101MOU-PUMC000155)
2. Human adipose-derived stem cells (hADSCs) (Procell, catalog number: CP-H202), provided as a cultured cell line
3. Primary mouse stromal vascular fraction (SVF), isolated from the inguinal white adipose tissue (iWAT) of two distinct age groups: postnatal day 8 (P8) pups and 8-week-old (8W) adult mice (purchased from Beijing SPF Biotechnology); cells from passages 1 or 2 were used for subsequent differentiation experiments
Reagents
1. Dulbecco's modified Eagle medium (DMEM), high glucose (Gibco, catalog number: 11995065)
2. Dulbecco's modified Eagle medium/Nutrient mixture F-12 (DMEM/F12) (Gibco, catalog number: 11330032)
3. Mesenchymal stem cell medium (MSCM) kit (ScienCell, catalog number: 7501, formulated with phenol red)
4. Newborn calf serum (NBCS) (Every Green, catalog number: 22011-8612)
5. Fetal bovine serum (FBS), heat-inactivated (Hyclone, catalog number: SH30406.05)
6. Penicillin-streptomycin (P/S) solution (Gibco, catalog number: 15140122)
7. GlutaMAX supplement (Gibco, catalog number: 35050061)
8. Phosphate-buffered saline (PBS), without Ca2+ and Mg2+ (Biosharp, catalog number: BL302A)
9. 0.25% Trypsin-EDTA solution (Gibco, catalog number: 25200056)
10. Dimethyl sulfoxide (DMSO), cell culture grade (Sigma-Aldrich, catalog number: D2650)
11. Dexamethasone (DEX) (Sigma-Aldrich, catalog number: D4902, suitable for cell culture)
12. 3-Isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, catalog number: I7018)
13. 5 mg/mL insulin (Macgene, catalog number: CC101)
14. 50 mM rosiglitazone (Macgene, catalog number: CH004)
15. 10 mM indomethacin (Solarbio, catalog number: II0100)
16. Ethanol (Thermo Fisher Scientific, catalog number: BP2818-500)
17. Isopropanol (Sigma-Aldrich, catalog number: 190764)
18. Oil Red O powder (Solarbio, catalog number: O8010)
19. 4% paraformaldehyde (PFA) solution (Solarbio, catalog number: P1110)
20. Collagenase I (Sigma-Aldrich, catalog number: C2674)
21. 0.1% Gelatin (OriCell, catalog number: GLT-11301)
22. Poly-D-Lysine (PDL) (Sigma-Aldrich, catalog number: P7280)
Solutions
1. Growth medium for 3T3-L1 preadipocytes (see Recipes)
2. Growth medium for hADSC cells (see Recipes)
3. Growth medium for primary mouse SVF (see Recipes)
4. 1 mM DEX solution (see Recipes)
5. 50 mM IBMX solution (see Recipes)
6. Adipogenic induction I medium for 3T3-L1 and hADSC cells (see Recipes)
7. Adipogenic induction I medium for primary mouse SVF (see Recipes)
8. Adipogenic induction II medium for 3T3-L1 and hADSC cells (see Recipes)
9. Adipogenic induction II medium for primary mouse SVF (see Recipes)
10. Adipogenic maintenance medium for 3T3-L1 and hADSC cells (see Recipes)
11. Adipogenic maintenance medium for primary mouse P8 SVF (see Recipes)
12. Adipogenic maintenance medium for primary mouse 8W SVF (see Recipes)
13. 0.3% Oil Red O stock solution (see Recipes)
14. Oil Red O working solution (see Recipes)
15. 1 mg/mL Collagenase I solution (see Recipes)
16. 1 mg/mL PDL solution (see Recipes)
17. 20 μg/mL PDL working solution (see Recipes)
18. Washing solution (see Recipes)
Recipes
1. Growth medium for 3T3-L1 preadipocytes
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 89% | 44.5 mL |
| NBCS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| Total | 100% | 50 mL |
Note: To prevent spontaneous differentiation during the maintenance phase, it is recommended to use newborn calf serum (NBCS) instead of fetal bovine serum (FBS). NBCS provides a lower baseline level of adipogenic-promoting factors, which helps maintain 3T3-L1 preadipocytes in an undifferentiated state during expansion, in accordance with the standard protocol for this cell line. For optimal stability, the prepared growth medium can be stored at 4 °C for up to 4 weeks.
2. Growth medium for hADSC cells
| Reagent | Final concentration | Volume |
|---|---|---|
| MSCM | 93% | 46.5 mL |
| FBS | 5% | 2.5 mL |
| P/S | 1% | 0.5 mL |
| Growth supplement | 1% | 0.5 mL |
| Total | 100% | 50 mL |
Note: All medium components are sourced from the Mesenchymal Stem Cell Medium kit and used per the manufacturer's protocol. For optimal stability, the prepared growth medium can be stored at 4 °C for up to 4 weeks.
3. Growth medium for primary mouse SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 88% | 44 mL |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| Total | 100% | 50 mL |
Note: For optimal stability, the prepared growth medium can be stored at 4 °C for up to 4 weeks.
4. 1 mM DEX (1,000× stock solution)
| Reagent | Stock concentration | Quantity |
|---|---|---|
| Ethanol | 2.54 mL | |
| DEX | 1 mM | 1 mg |
F.W: 392.4 g/mol.
Note: Dexamethasone stock solution is prepared in molecular biology–grade ethanol (≥99.5% purity). For optimal stability, sterilize the stock solution through a 0.22-μm filter before aliquoting. Store aliquots at -80 °C to avoid repeated freeze-thaw cycles.
5. 50 mM IBMX (100× stock solution)
| Reagent | Stock concentration | Quantity |
|---|---|---|
| DMSO | 1 mL | |
| IBMX | 50 mM | 11.5 mg |
F.W: 222.2 g/mol.
Note: For optimal stability, sterilize the stock solution through a 0.22-μm filter before aliquoting. Store aliquots at -80 °C to avoid repeated freeze-thaw cycles. If crystallization is observed during storage or handling, briefly warm the aliquot to 37 °C and vortex thoroughly until complete redissolution prior to use.
6. Adipogenic induction I medium for 3T3-L1 and hADSC cells
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 43.85 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| 1 mM DEX | 1 μM | 0.05 mL |
| 50 mM IBMX | 0.5 mM | 0.5 mL |
| 5 mg/mL insulin | 10 μg/mL | 0.1 mL |
| 50 mM rosiglitazone | 2.5 μM | 2.5 μL |
| Total | 50 mL |
Note: The switch from NBCS to FBS is critical for the initiation of adipogenic differentiation in 3T3-L1 preadipocytes. For optimal activity, prepare the adipogenic induction I medium fresh on the day of use by aseptic addition of all components to DMEM.
7. Adipogenic induction I medium for primary mouse SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 43.35 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| 1 mM DEX | 1 μM | 0.05 mL |
| 50 mM IBMX | 0.5 mM | 0.5 mL |
| 5 mg/mL insulin | 10 μg/mL | 0.1 mL |
| 50 mM rosiglitazone | 2.5 μM | 2.5 μL |
| Total | 50 mL |
Note: For optimal activity, prepare the adipogenic induction I medium fresh on the day of use by aseptic addition of all components to DMEM/F12.
8. Adipogenic induction II medium for 3T3-L1 and hADSC cells
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 44.4 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| 5 mg/mL insulin | 10 μg/mL | 0.1 mL |
| Total | 50 mL |
Note: For optimal activity, prepare the adipogenic induction II medium fresh on the day of use by aseptic addition of all components to DMEM.
9. Adipogenic induction II medium for primary mouse SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 43.9 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| 5 mg/mL insulin | 10 μg/mL | 0.1 mL |
| Total | 50 mL |
Note: For optimal activity, prepare the adipogenic induction II medium fresh on the day of use by aseptic addition of all components to DMEM/F12.
10. Adipogenic maintenance medium for 3T3-L1 and hADSC cells
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 89% | 44.5 mL |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| Total | 50 mL |
Note: For optimal stability, the prepared adipogenic maintenance medium can be stored at 4 °C for up to 4 weeks.
11. Adipogenic maintenance medium for primary mouse P8 SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 88% | 44 mL |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| Total | 50 mL |
Note: For optimal stability, the prepared adipogenic maintenance medium can be stored at 4 °C for up to 4 weeks.
12. Adipogenic maintenance medium for primary mouse 8W SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 43.99 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| 5 mg/mL insulin | 1 μg/mL | 0.01 mL |
| Total | 50 mL |
Notes: For optimal activity, prepare the adipogenic maintenance medium fresh on the day of use by aseptic addition of all components to the DMEM/F12.
13. 0.3% Oil Red O stock solution
| Reagent | Stock concentration | Quantity |
|---|---|---|
| Isopropanol | 100 mL | |
| Oil Red O | 0.3% | 0.3 g |
Note: To prepare the 0.3% Oil Red O stock solution, dissolve 0.3 g of powder in 100 mL of 100% isopropanol and stir the mixture overnight at room temperature to ensure complete dissolution. For optimal stability, store the solution at room temperature in a sealed container for up to 1 year.
14. Oil Red O working solution
| Reagent | Final concentration | Volume |
|---|---|---|
| ddH2O | 1 mL | |
| 0.3% Oil Red O | 0.2% | 2 mL |
Note: The Oil Red O working solution is prepared immediately before use by a 2:1 (v/v) dilution of the 0.3% stock solution with distilled water (ddH2O). The solution is then filtered twice through Whatman filter paper to remove crystalline aggregates and used within 2 h of preparation.
15. 1 mg/mL collagenase I solution
| Reagent | Final concentration | Volume |
|---|---|---|
| 1× PBS | 40 mL | |
| Collagenase I | 1 mg/mL | 50 mg |
| FBS | 20% | 10 mL |
| Total | 50 mL |
Note: All procedures use 1× calcium- and magnesium-free PBS. The Collagenase I used has been sourced from Clostridium histolyticum. To preserve stability, aliquot the stock solution to avoid repeated freeze-thaw cycles and store at -20 °C for up to 6 months.
16. 1 mg/mL PDL solution
| Reagent | Final concentration | Quantity |
|---|---|---|
| ddH2O | 5 mL | |
| PDL | 1 mg/mL | 5 mg |
Note: To preserve stability, aliquot the stock solution to avoid repeated freeze-thaw cycles and store at -20 °C for up to 6 months.
17. 20 μg/mL PDL working solution
| Reagent | Final concentration | Volume |
|---|---|---|
| ddH2O | 9.8 mL | |
| 1 mg/mL PDL | 20 μg/mL | 0.2 mL |
Note: For optimal cell attachment, the working solution should be prepared freshly before use and filter-sterilized. It can be stored at 4 °C for up to 1 month.
18. Washing solution
| Reagent | Final concentration | Volume |
|---|---|---|
| 1× PBS | 44.5 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| Total | 50 mL |
Note: The washing solution should be filter-sterilized (0.22 μm) and may be stored at 4 °C for up to 3 months.
Laboratory supplies
1. Cell culture dish, 60 mm × 15 mm (Corning, catalog number: 430196, tissue culture treated)
2. Cell culture dish, 100 mm × 20 mm (Corning, catalog number: 430167, tissue culture treated)
3. 6-well cell culture plate (Corning, catalog number: 3516, tissue culture treated)
4. 24-well cell culture plate (Corning, catalog number: 3524, tissue culture treated)
5. 96-well cell culture plate (Corning, catalog number: 3599, tissue culture treated)
6. 1.5 mL microtubes (Axygen, catalog number: MCT-150-C)
7. 15 mL centrifuge tube (Corning, catalog number: 430790)
8. 50 mL centrifuge tube (Corning, catalog number: 430828)
9. 10 mL serological pipettes (Corning, catalog number: 4488)
10. Cryogenic vials (Corning, catalog number: 430659)
11. 70 μm sterile cell strainers (Falcon, catalog number: 352350)
12. Parafilm (Amcor, catalog number: PM-996)
13. Micropipette tips, 0.5–10 μL (Axygen, catalog number: T-300)
14. Micropipette tips, 20–200 μL (Axygen, catalog number: T-200Y)
15. Micropipette tips, 100–1,000 μL (KIRGEN, catalog number: KG1313)
16. 0.22 μm syringe filter unit (Merck, catalog number: SLGPR33RB)
17. 10 mL Luer-LokTM syringe (BD, catalog number: 302149)
18. Isopropanol freezing container (Nalgene, catalog number: 5100-0001)
19. Whatman filter paper (Cytiva, catalog number: 1825-025)
Equipment
1. Inverted fluorescence microscope (Thermo Fisher Scientific, model: EVOS FL Auto 2)
2. Biosafety cabinet (BAKER, model: SG403A-HE-INT)
3. Laminar flow cabinet (ESCO, model: ACB-4A1)
4. Cell culture incubator (37 °C, 5% CO2) (Thermo Fisher Scientific, model: 371)
5. Centrifuge (Eppendorf, model: 5810R)
6. Water bath (Shanghai Yiheng, model: HWS-24)
7. Hemocytometer (Jiangsu, model: Improved Neubauer)
8. Inverted microscope (Leica, model: DMi8)
9. Surgical tools (scissors: 9.5 cm, straight, from Zhuoyue Medical Devices; forceps: 11 cm, straight or straight with a hook, from Zhuoyue Medical Devices)
10. Single-channel variable pipette, 0.1–2 μL (Rainin, model: Pipet-Lite XLS, catalog number: B735596768)
11. Single-channel variable pipette, 0.5–10 μL (Rainin, model: Pipet-Lite XLS, catalog number: B736643566)
12. Single-channel variable pipette, 20–200 μL (Rainin, model: Pipet-Lite XLS, catalog number: B735593478)
13. Single-channel variable pipette, 100–1,000 μL (Rainin, model: Pipet-Lite XLS, catalog number: B726316711)
14. -20 °C freezer (Haier, model: DW-40L508)
15. -80 °C freezer (Panasonic, model: MDF-682)
16. Vortex mixer (Kylin-Bell, model: VORTEX-5)
17. Analytic balance (Kunshan Youkeweite, model: CN-LQC5003)
18. LN2 dewar/storage system (DONGYA, model: YDS-110-290F)
19. Microplate reader (TECAN, model: Spark)
Software and datasets
1. ImageJ software (Fiji, version 1.53c, publicly available at https://fiji.sc/)
2. GraphPad Prism software (version 8.0.1 for Windows; GraphPad Software, Boston, Massachusetts, USA; https://www.graphpad.com/)
Procedure
文章信息
稿件历史记录
提交日期: Oct 22, 2025
接收日期: Dec 10, 2025
在线发布日期: Dec 25, 2025
出版日期: Jan 20, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Fan, J., Zhou, L., Geng, Y., Song, R., Wang, Y. and He, W. (2026). Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models. Bio-protocol 16(2): e5571. DOI: 10.21769/BioProtoc.5571.
分类
干细胞
细胞生物学 > 细胞分离和培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









