- EN - English
- CN - 中文
Isolation of Antigen-Specific Nanobodies From Synthetic Libraries Using a Protein Selection Strategy That Combines MACS-Based Screening of YSD and FLI-TRAP
发布: 2026年01月20日第16卷第2期 DOI: 10.21769/BioProtoc.5570 浏览次数: 34
评审: Willy R Carrasquel-UrsulaezBhanu JagilinkiAleksandra J. Wierzba
Abstract
Although protein–protein interactions (PPIs) are central to nearly all biological processes, identifying and engineering high-affinity intracellular binders remains a significant challenge due to the complexity of the cellular environment and the folding constraints of proteins. Here, we present a two-stage complementary platform that combines magnetic-activated cell sorting (MACS)-based yeast surface display with functional ligand-binding identification by twin-arginine translocation (Tat)-based recognition of associating proteins (FLI-TRAP), a bacterial genetic selection system for efficient screening, validation, and optimization of PPIs. In the first stage, MACS-based yeast display enables the rapid high-throughput identification of candidate binders for a target antigen from a large synthetic-yeast display library through extracellular interaction screening. In the second stage, an antigen-focused library is subcloned into the FLI-TRAP system, which exploits the hitchhiker export process of the Escherichia coli Tat pathway to evaluate binder–antigen binding in the cytoplasm. This stage is achieved by co-expressing a Tat signal peptide–tagged protein of interest with a β-lactamase-tagged antigen target, such that only binder–antigen pairs with sufficient affinity are co-translocated into the periplasm, thus rendering the bacterium β-lactam antibiotic resistant. Because Tat-dependent export requires fully folded and soluble proteins, FLI-TRAP further serves as a stringent in vivo filter for intracellular compatibility, folding, and stability. Therefore, this approach provides a powerful and cost-effective pipeline for discovering and engineering intracellular protein binders with high affinity, specificity, and functional expression in bacterial systems. This workflow holds promise for several applications, including synthetic biology and screening of theragnostic proteins and PPI inhibitors.
Key features
• Combines a single round of MACS enrichment with FLI-TRAP for high-throughput Nb discovery.
• Reduces time and resource demands compared to traditional workflows involving multiple rounds of MACS/FACS.
• Enables in vivo selection of high-affinity, functional binders via Tat-dependent export linked to β-lactam resistance, correlating binding affinity and solubility with antibiotic resistance.
Keywords: FLI-TRAPBackground
The biologics market is one of the fastest-growing sectors in the pharmaceutical industry, with over 100 antibody-based therapeutics now approved by the U.S. Food and Drug Administration (FDA) and/or the European Medicines Agency [1]. While full-length monoclonal antibodies (mAbs) remain the dominant form, other antibody derivatives have also gained regulatory approval, including fragment antigen-binding regions, single-chain variable fragments, and single-domain antibodies [also called variable domain of the heavy chain antibodies or nanobodies (Nbs)] [2]. Among these, Nbs derived from camelid species, such as camels, llamas, and alpacas, offer unique advantages due to their small size, high solubility, and exceptional stability. Since the FDA approved the first therapeutic Nb (caplacizumab) in 2019, Nbs have become increasingly valuable in both preclinical research and therapeutic development [3]. Their extended complementarity-determining region 3 enables them to access hidden or concave epitopes that are often inaccessible to conventional mAbs, while their small size facilitates microbial expression and simplifies downstream engineering [4].
Efficient and scalable platforms for Nb discovery are crucial for exploiting these properties. While being effective, conventional selection strategies, such as multiple rounds of magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS), are time-consuming and resource-intensive. In contrast, we recently demonstrated that a hybrid approach combining MACS-based yeast display with bacterial functional ligand-binding identification by twin-arginine translocation (Tat)-based recognition of associating proteins (FLI-TRAP) can significantly streamline the Nb discovery process [5,6]. We successfully isolated three highly specific Nbs against the catalytic domain of human proprotein convertase subtilisin/kexin type 9 (PCSK9) using only a single round of MACS pre-enrichment followed by a single round of FLI-TRAP selection [7]. This approach represents a significant improvement over traditional methods, which typically require 5–6 rounds of MACS and 1–2 rounds of FACS to achieve similar specificity. These findings highlight the high-throughput and cost-effective nature of combining MACS-based yeast display with FLI-TRAP (Figures 1 and 2).
FLI-TRAP leverages the unique capability of the Tat pathway in Escherichia coli, which exports fully folded and multimeric protein complexes from the cytoplasm to the periplasm. By fusing the Tat signal peptide of E. coli trimethylamine N-oxide reductase (ssTorA) to the Nb candidate (X) and a reporter protein [β-lactamase (Bla)] to the target antigen (Y), the successful protein–protein interaction (PPI) between X and Y enables the export of the complex into the periplasm, conferring resistance to β-lactam antibiotics such as ampicillin and carbenicillin. Notably, the degree of resistance correlates with binding affinity and/or solubility, allowing direct in vivo selection of high-affinity binders from focused libraries [5]. However, FLI-TRAP is limited by its reliance on soluble target proteins in the cytoplasm; insoluble proteins cannot be efficiently exported via the Tat pathway. This limitation can be addressed by expressing only the soluble fragments or functional domains of the target protein during screening [8,9].
Together, the combination of MACS-based yeast display for broad antigen-binding enrichment and FLI-TRAP for functional bacterial selection offers a powerful, rapid, and economical platform for Nb discovery. This two-step strategy reduces the need for iterative screening and enables efficient identification of binders with strong biological relevance, making it an attractive alternative to traditional methods.
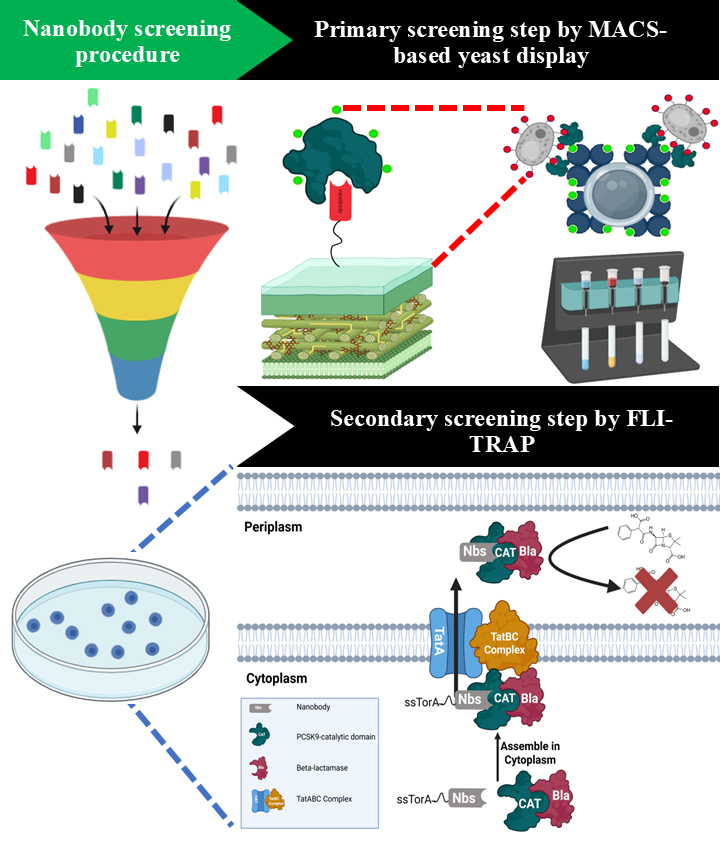
Figure 1. Schematic representation of the integrated platform for the isolation of PCSK9-specific nanobodies (Nbs) via combination of MACS-based yeast display and FLI-TRAP technique. A synthetic nanobody library is displayed on the surface of Saccharomyces cerevisiae BJ5465 and subjected to antigen-specific enrichment using MACS with recombinant PCSK9. This step selectively enriches yeast clones expressing Nbs with binding activity toward PCSK9. The enriched Nb population is subsequently transferred to a bacterial functional screening system based on functional ligand-binding identification by twin-arginine translocation (Tat)-based recognition of associating proteins (FLI-TRAP). In the FLI-TRAP system, Nb candidates are expressed in the cytoplasm of Escherichia coli as N-terminal fusions to the Tat signal peptide derived from trimethylamine N-oxide reductase (ssTorA), whereas PCSK9 is expressed as a fusion with the reporter enzyme β-lactamase (Bla). Productive Nb–PCSK9 interactions in the cytoplasm result in the formation of a stable protein complex that is specifically recognized by the Tat translocase. The Tat pathway mediates the translocation of fully folded proteins and protein complexes across the inner membrane into the periplasm; consequently, only correctly folded and sufficiently stable Nb–PCSK9–Bla complexes are exported. Periplasmic localization of Bla confers resistance to β-lactam antibiotics, thereby enabling direct in vivo selection of PCSK9-specific Nbs with favorable binding affinity and solubility based on bacterial survival under antibiotic selection pressure.
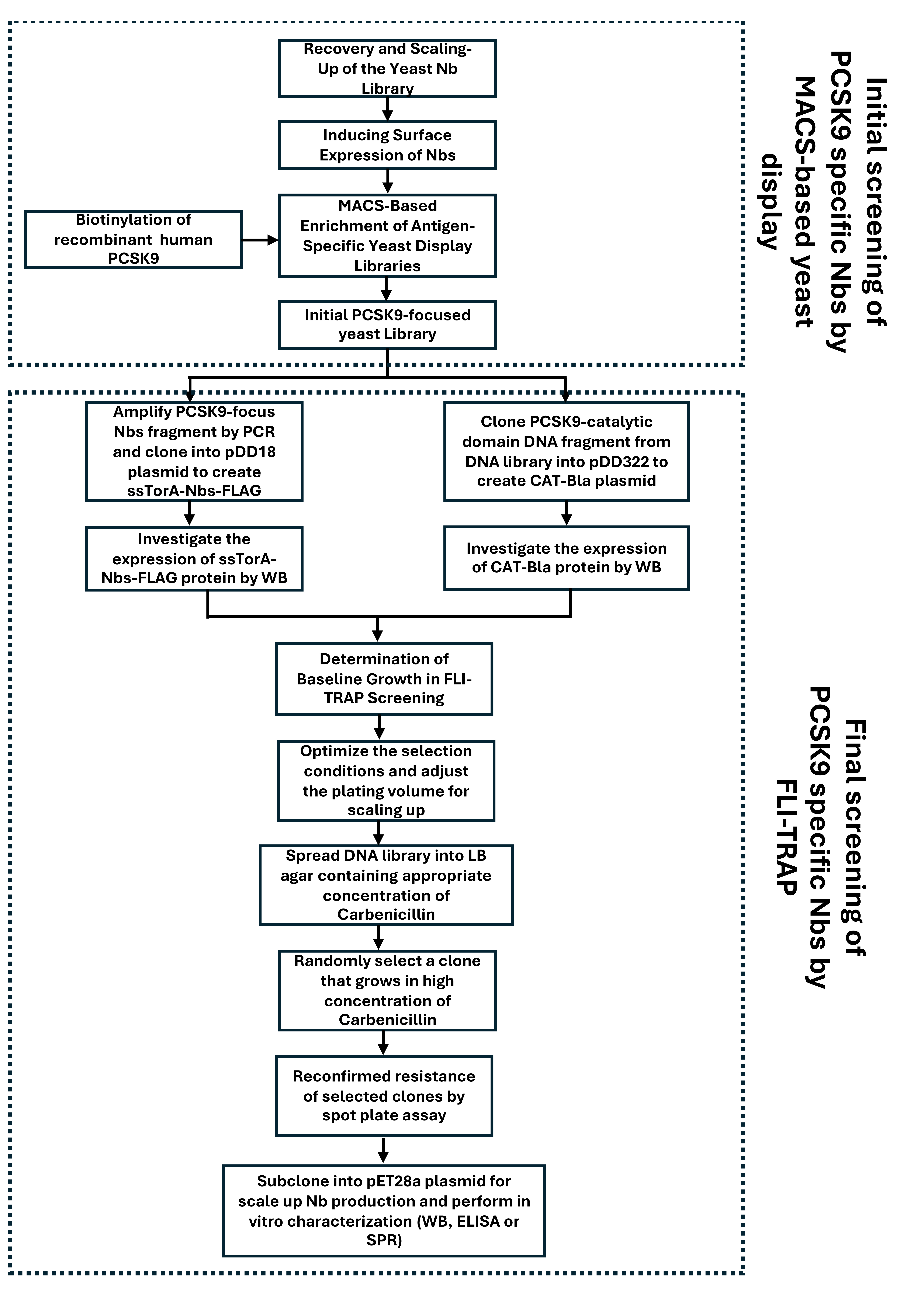
Figure 2. Critical steps in the combined magnetic-activated cell sorting (MACS)-based yeast display and functional ligand-binding identification by twin-arginine translocation (Tat)-based recognition of associating proteins (FLI-TRAP) workflow for screening PCSK9-specific nanobodies (Nbs).
Materials and reagents
Bacterial strains
1. Competent BL21(DE3) E. coli cells (New England Biolabs, catalog number: C2527H)
2. Competent NEB10β E. coli cells (New England Biolabs, catalog number: C3019H)
3. Saccharomyces cerevisiae strain BJ5465 (American Type Culture Collection, catalog number: 208289)
Reagents
1. Sodium citrate (Merck Millipore, CAS: 6132-04-03)
2. Yeast nitrogen base w/o amino acids (USBio, CAS: 38210000)
3. Glucose (Merck Millipore, CAS: 50-99-77)
4. Drop out mix synthetic minus tryptophan w/o yeast nitrogen base (USBio, catalog number: D9530)
5. Penicillin/streptomycin solution (Gibco, catalog number: 1TFS-1CC-15140122)
6. Galactose (Merck Millipore, CAS: 59-23-4)
7. Peptone (Himedia, catalog number: RM001-500G)
8. Yeast extract (Himedia, catalog number: RM027-500G)
9. Sodium chloride (Merck Millipore, CAS: 7647-14-5)
10. Agar (Himedia, CAS: GRM026)
11. Chloramphenicol (Merck Millipore, CAS: 56-75-7)
12. Kanamycin (Merck Millipore, CAS: 25389-94-0)
13. Carbenicillin (Sigma-Aldrich, CAS: 4800-94-6)
14. Arabinose (Merck Millipore, CAS: 5238-37-0)
15. Bacto-tryptone (Himedia, catalog number: M014-500G)
16. Dimethyl sulfoxide (DMSO) (Merck Millipore, CAS: 67-8-5)
17. Potassium chloride (Merck Millipore, CAS: 7447-40-7)
18. Magnesium sulfate (Merck Millipore, CAS: 7487-88-9)
19. Tween-20 (Merck Millipore, CAS: 9005-64-5)
20. Tris base (Merck Millipore, CAS: 77-68-1)
21. Sodium dodecyl sulfate (SDS) (Merck Millipore, CAS: 151-21-3)
22. 40% Acrylamide/Bis solution (Bio-Rad, CAS: 1610140)
23. Ammonium persulfate (APS) (Merck Millipore, CAS: 7727-54-0)
24. Bromophenol blue (Merck Millipore, CAS: 115-39-9)
25. Glycerol (Merck Millipore, CAS: 56-81-5)
26. β-mercaptoethanol (Merck Millipore, CAS: 60-24-2)
27. Ethylenediaminetetraacetic acid (EDTA) (Merck Millipore, CAS: 60-00-04)
28. Tetramethylethylenediamine (TEMED) (Merck Millipore, CAS: 110-18-9)
29. EZ-LinkTM Sulfo-NHS-Biotin (Thermo ScientificTM, catalog number: 21217)
30. Sodium phosphate dibasic (Merck Millipore, CAS: 7558-79-4)
31. Potassium phosphate monobasic (Merck Millipore, CAS: 7778-77-0)
32. Acetic acid (Merck Millipore, CAS: 64-19-7)
33. HA Tag recombinant mouse monoclonal antibody (2-2.2.14), Alexa FluorTM Plus 488 (Thermo Fischer Scientific, catalog number: 740005MP48820UG)
34. HA Tag recombinant mouse monoclonal antibody (2-2.2.14), Alexa FluorTM Plus 647 (Thermo Fischer Scientific, catalog number: 740005MP64720UG)
35. Zymolyase (Zymo Research, catalog number: E1004)
36. Quick StartTM Bradford protein assay (Bio-Rad, catalog number: 5000201)
37. Hydrochloric acid (HCl) (Merck Millipore, CAS: 258148)
38. Sodium hydroxide (NaOH) (Merck Millipore, CAS: 221465)
39. HRP Anti-HA tag antibody (Abcam, catalog number: 173826)
40. EasySepTM Direct Human PBMC Isolation kit (STEMCELL Technologies, catalog number: 19654)
41. Clarity Max Western ECL substrate (Bio-Rad, catalog number: 1705062)
42. Recombinant Human PCSK9 protein (His & AVI Tag), biotinylated, HPLC-verified (Sino Biological, catalog number: 29698-H27H-B)
43. xbaI restriction enzyme (Bio-Rad, catalog number: R0145S)
44. SalI-HF restriction enzyme (Bio-Rad, catalog number: R3138S)
45. AvrII restriction enzyme (Bio-Rad, catalog number: R0174S)
46. NoTI-HF restriction enzyme (Bio-Rad, catalog number: R3189S)
47. NucleoSpin Plasmid Mini kit for plasmid DNA (MACHEREY-NAGEL, catalog number: 740588.50)
48. NucleoSpin Gel and PCR Clean-up Mini kit for gel extraction or PCR clean up (MACHEREY-NAGEL, catalog number: 740609.50)
49. T4 DNA ligase (Bio-Rad, catalog number: M0202S)
50. Anti-FLAG-HRP (HRP Anti-DDDDK tag, binds to FLAG® tag sequence) antibody (M2) (Abcam, catalog number: ab49763)
51. Anti-Beta lactamase antibody (Abcam, catalog number: ab12251)
52. Goat anti-mouse IgG secondary antibody (HRP) (Sino Biological, catalog number: SSA007)
53. Absolute ethanol (Merck Millipore, CAS: 64-17-5)
54. Blotting-grade blocker (Bio-Rad, catalog number: 1706404)
Solutions
1. Yglc4.5–Trp medium (see Recipes)
2. Yglc–Gal pH 6.0 (see Recipes)
3. Luria-Bertani (LB) broth (see Recipes)
4. LB agar (see Recipes)
5. Chloramphenicol solution (see Recipes)
6. Kanamycin solution (see Recipes)
7. Carbenicillin solution (see Recipes)
8. Arabinose solution (see Recipes)
9. Super optimal broth (SOB) medium (see Recipes)
10. Phosphate buffer saline (PBS) (see Recipes)
11. Western blot blocking buffer (see Recipes)
12. Phosphate buffer saline with Tween-20 (PBST) (see Recipes)
13. 0.5 M Tris pH 6.8 solution (see Recipes)
14. 1.5 M Tris pH 8.8 solution (see Recipes)
15. 10% SDS solution (see Recipes)
16. 10% ammonium persulfate (APS) (see Recipes)
17. 6× Laemmli sample loading buffer (see Recipes)
18. 12% SDS-polyacryalmide gel (see Recipes)
19. 6% SDS-polyacrylamide gel (see Recipes)
20. 50× Tris-acetate-EDTA buffer (TAE) (see Recipes)
21. Cell lysis buffer (see Recipes)
22. 10% APS (see Recipes)
23. Yeast extract peptone dextrose agar (see Recipes)
Recipes
1. Yglc4.5–Trp medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium citrate | 80 mM | 20.6448 g |
| Yeast nitrogen base w/o amino acids | 6.7 g/L | 6.7 g |
| Glucose | 2% w/v | 20 g |
| Drop out mix synthetic minus tryptophan w/o yeast nitrogen base | 3.8 g/L | 3.8 g |
| Penicillin/streptomycin solution | 1× | 10 mL |
| H2O | n/a | To 1,000 mL |
Adjust pH to 4.5 with 1 N HCl and sterilize by autoclaving.
2. Yglc–Gal pH 6.0
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Yeast nitrogen base w/o amino acids | 6.7 g/L | 6.7 g |
| Galactose | 2% w/v | 20 g |
| Drop out mix synthetic minus tryptophan w/o yeast nitrogen base | 3.8 g/L | 3.8 g |
| Penicillin/streptomycin solution | 1× | 10 mL |
| H2O | n/a | To 1,000 mL |
Adjust pH to 4.5 with 1 N HCl and sterilize by autoclaving.
3. LB broth
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Peptone | 10 g/L | 10 g |
| Yeast extract | 10 g/L | 10 g |
| Sodium chloride | 5 g/L | 5 g |
| H2O | n/a | To 1,000 mL |
Adjust pH to 7.4 with 1 N HCl and sterilize by autoclaving.
4. LB agar
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Peptone | 10 g/L | 10 g |
| Yeast extract | 10 g/L | 10 g |
| Sodium chloride | 5 g/L | 5 g |
| Agar | 15 g/L | 15 g |
| H2O | n/a | To 1,000 mL |
Adjust pH to 7.4 with 1 N HCl and sterilize by autoclaving.
5. Chloramphenicol solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Chloramphenicol | 34 g/L | 340 mg |
| Absolute ethanol | n/a | To 10 mL |
Sterilize by filtering with a 0.22 μm filter. Aliquot in a 1 mL sterile microtube and store at -20 °C.
6. Kanamycin solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Kanamycin | 50 g/L | 500 mg |
| H2O | n/a | To 10 mL |
Sterilize by filtering with a 0.22 μm filter. Aliquot in a 1 mL microtube and store at -20 °C.
7. Carbenicillin solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Carbenicillin | 100 g/L | 1 g |
| H2O | n/a | To 10 mL |
Sterilize by filtering through a 0.22 μm filter. Aliquot 1 mL into sterile amber microtubes and store at -20 °C.
8. Arabinose solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Arabinose | 20% w/v | 20 g |
| H2O | n/a | To 100 mL |
Sterilize by filtering through a 0.22 μm filter. Aliquot 1 mL into sterile microtubes and store at -20 °C.
9. SOB medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Bacto-tryptone | 20 mg/mL | 20 g |
| Yeast extract | 10 mg/mL | 10 g |
| Sodium chloride | 5 mg/mL | 5 g |
| Potassium chloride | 2.5 mM | 0.186 g |
| Magnesium sulfate | 20 mM | 2.4 g |
| H2O | n/a | To 1,000 mL |
Adjust the pH to 7.4 with 1 N HCl and sterilize by autoclaving.
10. PBS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride | 0.137 M | 8 g |
| Potassium chloride | 2.7 mM | 0.2 g |
| Sodium phosphate dibasic | 10 mM | 1.44 g |
| Potassium phosphate monobasic | 1.8 mM | 0.245 g |
| H2O | n/a | To 1,000 mL |
Adjust pH to 7.4 with 1 N HCl and sterilize by autoclaving.
11. Western blot blocking buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride | 0.137 M | 8 g |
| Potassium chloride | 2.7 mM | 0.2 g |
| Sodium phosphate dibasic | 10 mM | 1.44 g |
| Potassium phosphate monobasic | 1.8 mM | 0.245 g |
| Blotting-grade blocker | 5% w/v | 50 g |
| H2O | n/a | To 1,000 mL |
Adjust pH to 7.4 with 1 N HCl and sterilize by autoclaving.
12. PBST
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride | 0.137 M | 8 g |
| Potassium chloride | 2.7 mM | 0.2 g |
| Sodium phosphate dibasic | 10 mM | 1.44 g |
| Potassium phosphate monobasic | 1.8 mM | 0.245 g |
| Tween-20 | 0.1% v/v | 1 mL |
| H2O | n/a | To 1,000 mL |
Adjust pH to 7.4 with 1 N HCl and sterilize by autoclaving.
13. 0.5 M Tris pH 6.8
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris base | 0.5 M | 18.2 g |
| H2O | n/a | To 100 mL |
Adjust pH to 6.8 with 1 N HCl and sterilize by autoclaving.
14. 1.5 M Tris pH 8.8
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris base | 0.5 M | 12.1 g |
| H2O | n/a | To 100 mL |
Adjust pH to 8.8 with 1 N HCl and sterilize by autoclaving.
15. 10% SDS solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| SDS | 10% w/v | 10 g |
| H2O | n/a | To 100 mL |
16. 10% APS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| APS | 10% w/v | 10 g |
| H2O | n/a | To 100 mL |
17. 6× Laemmli sample loading buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| SDS | 10% w/v | 1 g |
| Bromophenol blue | 0.01% v/v | 10 mg |
| Glycerol | 50% w/v | 5 mL |
| Tris base | 0.5 M | 0.6 g |
| β-mercaptoethanol | 10% v/v | 1 mL |
| H2O | n/a | To 10 mL |
18. 12% SDS polyacrylamide gel
| Reagent | Quantity or volume |
|---|---|
| 40% acrylamide/bis solution | 2.4 mL |
| 1.5 M Tris pH 8.8 | 2 mL |
| 10% SDS | 80 μL |
| 10% APS | 80 μL |
| TEMED | 8 μL (0.1% v/v) |
| H2O | To 8 mL |
19. 6% SDS polyacrylamide gel
| Reagent | Quantity or volume |
|---|---|
| 40% acrylamide/bis solution | 0.75 mL |
| 1.5 M Tris pH 8.8 | 1.25 mL |
| 10% SDS | 50 μL |
| 10% APS | 50 μL |
| TEMED | 5 μL (0.1% v/v) |
| H2O | To 5 mL |
20. 50× TAE
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris base | 2 M | 242.2 g |
| Acetic acid | 1 M | 57.1 mL |
| EDTA | 50 mM | 18.612 g |
| H2O | n/a | To 1,000 mL |
21. Cell lysis buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride | 0.137 M | 8 g |
| Potassium chloride | 2.7 mM | 0.2 g |
| Sodium phosphate dibasic | 10 mM | 1.44 g |
| Potassium phosphate monobasic | 1.8 mM | 0.245 g |
| SDS | 1% w/v | 10 g |
| H2O | n/a | To 1,000 mL |
Adjust pH to 7.4 with 1 N HCl and sterilize by autoclaving.
22. 10% APS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| APS | 10% w/v | 10 ml |
| H2O | n/a | To 100 mL |
23. Yeast extract peptone dextrose agar (YPD)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Yeast extract | 10 g/L | 10 g |
| Peptone | 20 g/L | 20 g |
| Glucose | 20 g/L | 20 g |
| Agar | 15 g/L | 15 g |
| H2O | n/a | To 1000 mL |
Laboratory supplies
1. DURAN® culture flask, Erlenmeyer shape, straight neck, 2,000 mL (DWK Life Sciences, catalog number: 217716309)
2. DURAN® culture flask, Erlenmeyer shape, straight neck, 200 mL (DWK Life Sciences, catalog number: 217713209)
3. Falcon® 100 mm × 15 mm not TC-treated bacteriological Petri dish (Corning, catalog number: 351029)
4. Axygen® 1.5 mL snap-lock microcentrifuge tube, polypropylene, clear, sterile (Corning, catalog number: MCT-150-C-S)
5. Corning® polycarbonate 1–2 mL cryogenic vial storage box, holds 81 vials (Corning, catalog number: 431119)
6. Corning® 2 mL external threaded polypropylene cryogenic vial with 1D and 2D bar codes (Corning, catalog number: 8671)
7. Corning® DeckWorks 0.1–10 µL low-binding pipette tips, graduated, hinged racks, natural, nonsterile, polypropylene (Corning, catalog number: 4147)
8. Corning® DeckWorks 1–200 µL low-binding pipette tips, graduated, hinged racks, natural, nonsterile, polypropylene (Corning, catalog number: 4148)
9. Corning® DeckWorks 1–300 µL low-binding pipette tips, graduated, hinged racks, natural, nonsterile, polypropylene (Corning, catalog number: 4149)
10. Polyvinylidene difluoride (PVDF) western blotting membrane (Merck Millipore, CAS: 03010040001)
11. 0.22 µm filter membranes, nitrocellulose (Merck Millipore, CAS: Z358657)
12. Gene pulser/MicroPulser electroporation cuvettes, 0.1 cm gap (Bio-Rad, catalog number: 1652083)
Equipment
1. Incubator shaker (RADOBIO, catalog number: MS350T)
2. Multiskan SkyHigh microplate spectrophotometer (Thermo Fischer Scientific, catalog number: A51119500C)
3. SorvallTM LegendTM Micro 21R microcentrifuge (Thermo Fischer Scientific, catalog number: 75002544)
4. Pipet-Lite LTS Pipette L-2XLS+ manual single-channel pipette, 0.1–2 μL (Mettler Toledo, catalog number: 17014393)
5. Pipet-Lite LTS Pipette L-20XLS+ manual single-channel pipette, 2–20 μL (Mettler Toledo, catalog number: 17014392)
6. Pipet-Lite LTS Pipette L-20XLS+ manual single-channel pipette, 10–100 μL (Mettler Toledo, catalog number: 17014384)
7. Pipet-Lite LTS Pipette L-20XLS+ manual single-channel pipette, 100–1,000 μL (Mettler Toledo, catalog number: 17014382)
8. Pipet-Lite Multi Pipette L8-50XLS+ manual single-channel pipette, 5–50 μL (Mettler Toledo, catalog number: 17013804)
9. TSX Universal Series general purpose ultra-low freezers (Thermo Fischer Scientific, catalog number: TSX70086FA)
10. Attune Xenith flow cytometer (Thermo Fischer Scientific, catalog number: A59358)
11. Ultrasonic homogenizer (BUENO BIOTECH, model: BEM-150A)
12. PrecisionTM general purpose baths (Thermo Fischer Scientific, catalog number: TSGP02)
13. EasySepTM magnet (STEMCELL Technologies, catalog number: 18000)
14. ChemiDoc XRS+ system (Bio-Rad, catalog number: 1708265)
15. HerathermTM general protocol microbiological incubators (Thermo Fischer Scientific, catalog number: 51028063)
16. MicroPulser electroporator (Bio-Rad, catalog number: 1652100)
Procedure
文章信息
稿件历史记录
提交日期: Sep 29, 2025
接收日期: Dec 7, 2025
在线发布日期: Dec 30, 2025
出版日期: Jan 20, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Thaiprayoon, A., Chantarasorn, Y., Oonanant, W., Kasorn, A., Longsompurana, P., Tapaneeyakorn, S., Riangrungroj, P., Loison, F., Kruse, A. C., DeLisa, M. P. and Waraho-Zhmayev, D. (2026). Isolation of Antigen-Specific Nanobodies From Synthetic Libraries Using a Protein Selection Strategy That Combines MACS-Based Screening of YSD and FLI-TRAP. Bio-protocol 16(2): e5570. DOI: 10.21769/BioProtoc.5570.
分类
生物工程 > 生物医学工程
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link