- EN - English
- CN - 中文
A Highly Efficient siRNA Transfection Method in Primary Cultured Cortical Neurons
发布: 2026年01月20日第16卷第2期 DOI: 10.21769/BioProtoc.5567 浏览次数: 18
评审: Olga KopachAnonymous reviewer(s)
Abstract
Transfecting neurons remains technically challenging due to their sensitivity. Conventional methods, such as Lipofectamine 2000 or Lipofectamine RNAiMAX, often result in significant cytotoxicity, which limits their utility. Although lentiviral transfection offers high efficiency, it is hindered by high costs and complex procedures. This experiment employs a small interfering RNA (siRNA)-specific transfection reagent from the Kermey company. This reagent is a novel nanoparticle-based lipid material designed for the efficient delivery of oligonucleotides, including siRNA, into a wide range of cell types. Its efficacy in achieving high transfection efficiency in neurons, however, has not yet been established. After several days of in vitro neuronal culture, researchers can perform a simple transfection procedure using this reagent to achieve robust transfection efficiency. Notably, the protocol does not require medium replacement 6–8 h post-transfection, streamlining the workflow and minimizing cellular stress.
Key features
• Based on Kermey’s siRNA-specific transfection reagent, we present a method for efficient in vitro transfection of siRNA into primary cultured mouse cortical neurons.
• No observable adverse effects are detected in the transfected neurons during the entire experiment.
• This method enables consistent and efficient knockdown of the target protein.
• Phosphoglycerate dehydrogenase (PHGDH) siRNA and siNC (negative control) siRNA can be transfected into neuronal cells after 72 h of in vitro culture.
Keywords: NeuronGraphical overview
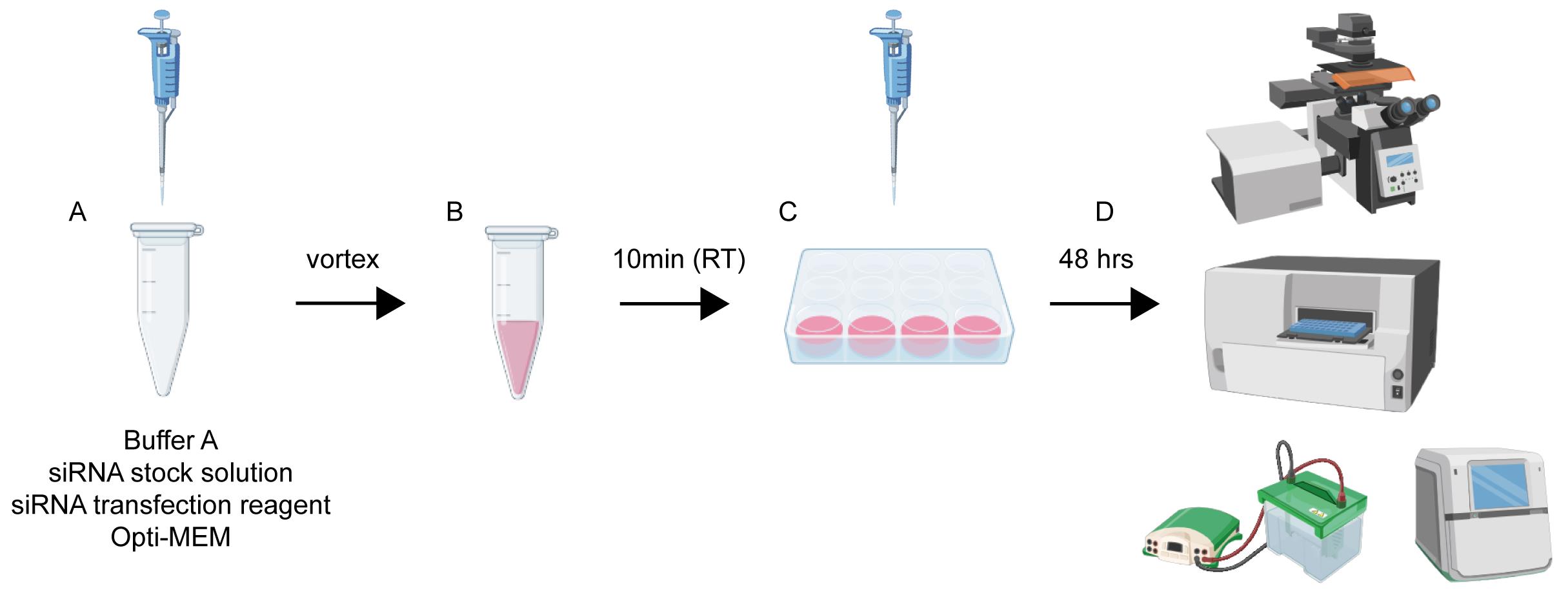
Workflow and timeline for neuronal transfection. (A) Reagents are sequentially added to a microcentrifuge tube in volumes appropriate for the specific culture plate. (B) The mixture from step A is vortexed thoroughly and incubated at room temperature for 10 min to allow complex formation. (C) The transfection mixture is then added dropwise and evenly to the neuronal culture medium. (D) Forty-eight hours post-transfection, cells are ready for downstream assays, including western blotting, immunofluorescence staining, and cell viability analysis.
Background
Investigating neuronal survival and function is essential for understanding the structure, development, and function of the nervous system. It is also critical for addressing neurological disorders such as neurodegenerative diseases, stroke, and neuropsychiatric conditions [1,2]. Effective gene-targeting strategies are vital to elucidate gene functions, clarify causal relationships and underlying molecular signaling pathways, and develop novel therapeutic approaches to modulate neuronal death and function.
Currently, small interfering RNA (siRNA)-mediated gene knockdown is widely used in various cell types and cell lines in vitro and even in vivo through specialized delivery techniques [3]. However, delivering siRNA into primary neuronal cells remains a significant technical challenge and a costly endeavor [4]. Primary neurons, due to their post-mitotic nature, are inherently more difficult to transfect than proliferating cells and are highly susceptible to cell death [5]. Presently, viral vector-mediated siRNA delivery is commonly employed for neurons and offers reliable efficacy along with long-term stability. However, this approach involves complex procedures, high costs, and potential cytotoxicity [6,7]. In contrast, conventional transfection methods, such as Lipofectamine 2000 or RNAiMAX, frequently induce neuronal toxicity, compromising cell viability and potentially confounding experimental outcomes. Moreover, these reagents typically yield low transfection efficiency in primary neurons [7]. Additionally, nucleofection is only applicable to neurons on the day of plating, when they are in suspension and have not yet extended neurites [8]. Given these limitations, there is a pressing need to develop safer, more efficient, and neuron-compatible transfection strategies that enable robust siRNA delivery in primary cultured neurons without compromising their physiological integrity.
To address these limitations, we developed an optimized transfection protocol for mouse primary neurons using Kermey’s siRNA-specific transfection reagent. This newly developed and commercialized product is a novel nano-lipid material designed for efficient oligonucleotide delivery into various cell types. Comparative analyses with conventional reagents, such as Lipofectamine 2000 and RNAiMAX, demonstrate that this new method achieves superior siRNA delivery efficiency with minimal cytotoxicity, robust target gene knockdown, and excellent preservation of neuronal viability and physiological characteristics. These advantages are likely attributable to the unique design of the nano-lipid material, which minimizes immune activation and membrane-related cellular stress. This advancement provides researchers with a reliable, cost-effective tool for conducting mechanistic studies in primary neuronal systems.
Materials and reagents
Biological materials
1. C57BL/6L mouse [Ji’nan Pengyue Laboratory Animal Breeding Co., Ltd. (China), origin: Ji’nan Shandong, China]
Reagents
1. Poly-D-lysine (Sigma, catalog number: P6407-10X5MG)
2. NeurobasalTM medium (1×) (Gibco, catalog number: 21103049)
3. B-27 supplement (50×) (Gibco, catalog number: 17504044)
4. siRNA transfection reagent (Kermey, catalog number: MLR0201)
5. LipofectamineTM RNAiMAX transfection reagent (Thermo Fisher Scientific, catalog number: 13778150)
6. GlutaMAXTM (100×) (Gibco, catalog number: 35050061)
7. Dulbecco’s modified Eagle medium (DMEM) basic (1×) (Gibco, catalog number: 11995065)
8. Penicillin-streptomycin liquid (100×) (Solarbio, catalog number: P1400)
9. Phosphate-buffered solution (PBS), 0.01 M (powder, pH 7.2–7.4) (Solarbio, catalog number: P1010)
10. Opti-MEM (1×) (Gibco, catalog number: 31985047)
11. Fluorescent dye-labeled siRNA negative control (GenePharma)
12. Standard negative control (siNC) (GenePharma, sequence: UUCUCCGAACGUGUCACGUtt)
13. Custom siRNA targeting PHGDH (GenePharma, sequence: UCGGCAGAAUUGGAAGAGAtt)
14. Triton X-100 (Solarbio, catalog number: T8200)
15. Albumin (bovine fraction V) (BSA) (MeilunBio, CAS: 9048-46-8)
16. DAPI (Solarbio, catalog number: S2110)
17. Paraformaldehyde (PFA), 4% (Solarbio, catalog number: P1110)
18. Primary antibody used in this study: PHGDH (ProteinTech, catalog number: 14719-1-AP)
19. Second antibody used in this study: HRP-conjugated Affinipure goat anti-rabbit IgG (H+L) (SA00001-2) (ProteinTech)
20. CCK8 Assay kit (MeilunBio, CAS: MA0218)
21. Fetal bovine serum (FBS) (Sigma, catalog number: F0193-500ML)
Solutions
1. Triton X-100, 0.5% (see Recipes)
2. BSA, 5% (see Recipes)
3. Neurobasal medium (see Recipes)
4. Trypsin, 0.025% (see Recipes)
5. Complete medium (see Recipes)
6. PDL 10× (see Recipes)
7. PDL 1× (see Recipes)
Recipes
1. Triton X-100, 0.5%
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Triton X-100, 100% | 0.5% (v/v) | 250 μL |
| PBS | n/a | 49.75 mL |
| Total | n/a | 50 mL |
2. BSA, 5%
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BSA | 1/50 | 5 g |
| PBS | n/a | 100 mL |
| Total | n/a | 100 mL |
3. Neurobasal medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NeurobasalTM medium | 48/50 | 48 mL |
| B-27 Supplement | 1/50 | 1 mL |
| GlutaMAXTM | 1/100 | 0.5 mL |
| Penicillin-streptomycin, liquid | 1/100 | 0.5 mL |
| Total | n/a | 50 mL |
4. Trypsin, 0.025%
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Trypsin, 0.25% | 1/10 | 5 mL |
| DMEM | 9/10 | 45 mL |
| Total | n/a | 50 mL |
5. Complete medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM | 44/50 | 44 mL |
| FBS | 1/10 | 5 mL |
| GlutaMAXTM | 1/100 | 0.5 mL |
| Penicillin-streptomycin, liquid | 1/100 | 0.5 mL |
| Total | n/a | 50 mL |
6. PDL 10×
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Poly-D-lysine | n/a | 5 mg |
| PBS | n/a | 50 mL |
| Total | n/a | 50 mL |
7. PDL 1×
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PDL, 10× | 1/10 | 1 mL |
| PBS | n/a | 9 mL |
| Total | n/a | 10 mL |
Laboratory supplies
1. 24-well tissue culture plate (JET, catalog number: TCP011124)
2. 24-well round coverslip (WHB, catalog number: WHB-24-CS)
3. 50 mL centrifuge tube (LABSELECT, catalog number: CT-012-50)
4. 15 mL centrifuge tube (LABSELECT, catalog number: CT-002-15A)
5. 1.5 mL centrifuge tube (LABSELECT, catalog number: MCT-001-150)
6. 0.22 μm PES syringe filter (Biosharp, catalog number: BS-PES-22)
7. 50 mL syringe (Beyotime, catalog number: FS850-20pcs)
8. 200 μL RNase-free pipette (Biosharp, catalog number: BS-200-TRS)
9. 10 μL RNase-free pipette (Biosharp, catalog number: BS-RT-10)
Equipment
1. Dissection microscope (RWD, model: 77001S)
2. Dissection tools: forceps (RWD, model: FC4001R-11) and iris scissors (RWD, model: S12003-09)
3. Biosafety cabinet (Esco, model: AC2-4S1)
4. CO2 incubator (Thermo Scientific, model: Forma Steri-Cycle i160 CR)
5. SpectraMax ABS (Molecular Devices, USA)
6. Low-speed desktop centrifuge (BAIOU, model: TDL-320)
7. Amersham ImageQuantTM 800 GxP (Cytiva, model: 29653452)
8. Confocal microscope (Nikon, model: Nikon C2)
Software and datasets
1. Image J [NIH, Java 1.8.0_345 (64-bit)]
Procedure
文章信息
稿件历史记录
提交日期: Oct 9, 2025
接收日期: Dec 10, 2025
在线发布日期: Dec 26, 2025
出版日期: Jan 20, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wang, X., Li, Y., Sun, X., Cui, Y. and Zhang, Z. (2026). A Highly Efficient siRNA Transfection Method in Primary Cultured Cortical Neurons. Bio-protocol 16(2): e5567. DOI: 10.21769/BioProtoc.5567.
分类
神经科学 > 细胞机理
细胞生物学 > 基于细胞的分析方法 > 基因表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link