- EN - English
- CN - 中文
A Simple Protocol for Periodic Live Cell Observation of Flagellate Stages in the Lichen Alga Trebouxia
发布: 2026年01月20日第16卷第2期 DOI: 10.21769/BioProtoc.5566 浏览次数: 36
评审: Dennis J NürnbergAnonymous reviewer(s)

相关实验方案

灯台南洋杉(南洋杉科)中原胚团的分离及生长和形态测定
Jackellinne Caetano Douétts-Peres [...] Claudete Santa-Catarina
2016年12月05日 9584 阅读
Abstract
Flagellate stages of green microalgae such as Trebouxia are only partially characterised, with recent evidence suggesting that they are involved in both sexual and asexual reproduction. Conventional methods based on fixed samples in light, confocal, or electron microscopy provide only static observations and prevent real-time monitoring of living cells. To overcome this limitation, we have developed a simple and cost-effective protocol for observing Trebouxia flagellate cells over several days by coating microscopy slides with Bold’s basal medium. The method preserves cell viability and allows repeated imaging of motile cells in the same areas so that their behaviour and development can be continuously observed. In this way, qualitative observations, such as flagellate cell release, motility, and gamete fusion, can be combined with quantitative analyses of cell morphology. The protocol has proven to be robust and reproducible and was applied to several Trebouxia species. Compared to existing techniques, it allows the monitoring of dynamic processes and provides a powerful tool to study specific life stages not only in Trebouxia but also in other unicellular and colonial green algae.
Key features
• This protocol allows real-time monitoring over several days of Trebouxia flagellate cells with standard light microscopy.
• This protocol preserves cell viability and motility for repeated daily observations of the same cell groups.
• This protocol is simple, low-cost, and adaptable to other motile algal cells.
• This protocol is based on the methodology described in [1], where it was originally applied and validated.
Keywords: Light microscopyGraphical overview
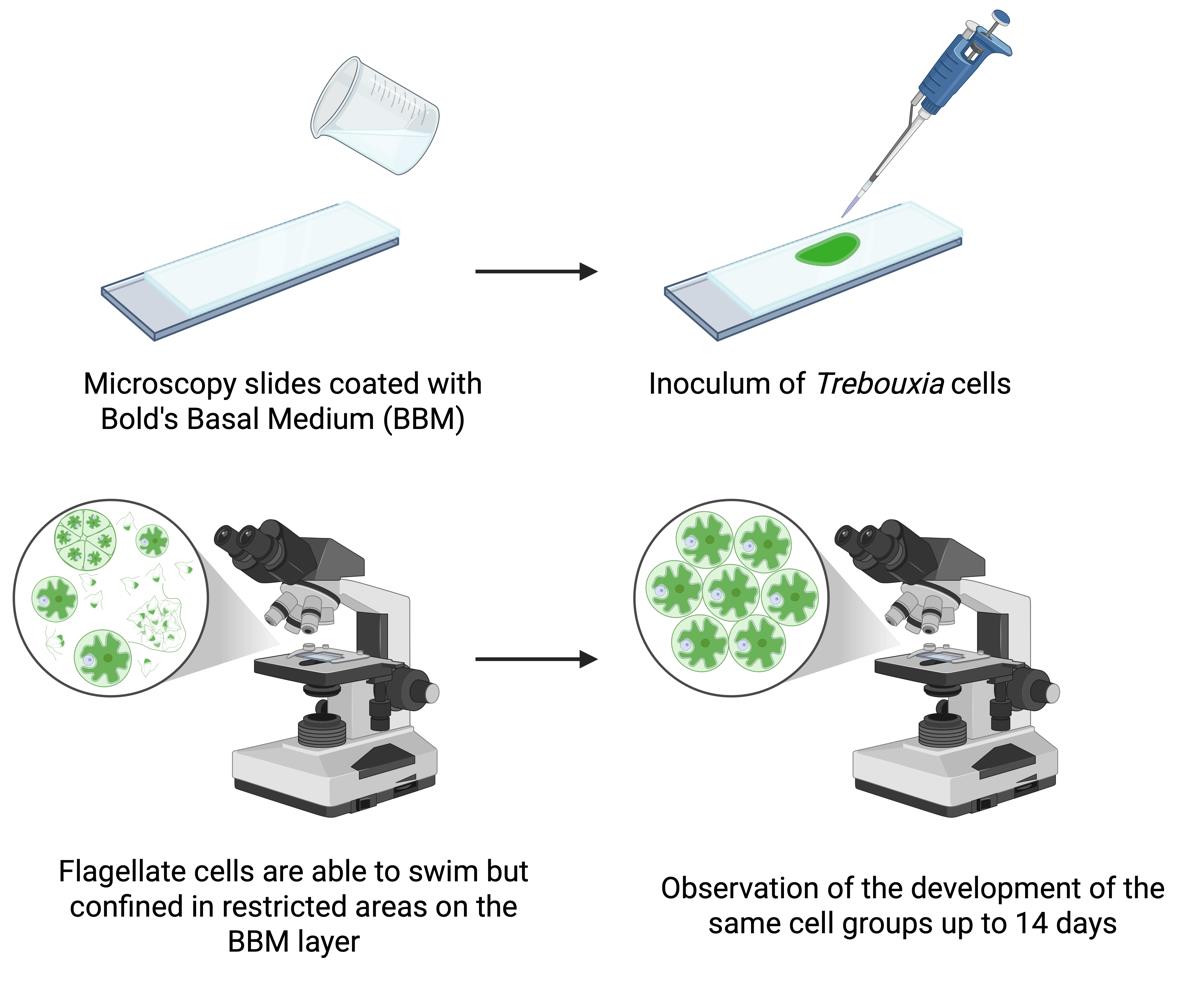
Workflow for observation of flagellate cells of Trebouxia over several days
Background
Trebouxia Puymaly (Trebouxiophyceae, Chlorophyta) is a genus of unicellular, aero-terrestrial green microalgae whose representatives are the photosynthetic partner (i.e., photobiont) to about 50% of all lichen species [2]. The study of Trebouxia in culture is of fundamental importance as key traits for species identification, such as chloroplast morphology or specific stages of the life cycle, are difficult or impossible to observe in the lichenised form [3]. Trebouxia can reproduce asexually via autospores and aplanospores (non-motile) and via flagellate zoospores (motile). While the non-motile stages of the life cycle have been extensively studied for their taxonomic traits [4,5], the motile stages have been neglected over the years, so information on sexual reproduction in Trebouxia is limited. Sexual reproduction, which is common in other green algae such as Chlorophyceae and Ulvophyceae [6], is considered rare or absent in Trebouxiophyceae [7]. However, recent genome and transcriptome analyses have demonstrated the presence of meiotic genes in some representatives, suggesting possible sexual reproduction [6,8]. A recent study on Trebouxia lynniae [9], using flow cytometry and confocal techniques, showed that mature vegetative cells have twice the DNA content of flagellate cells, which were hence interpreted as gametes. This indicates the presence of sexual reproduction and challenges the current knowledge that flagellate cells only play a role in asexual reproduction. Further studies are thus clearly needed. A major limitation of previous studies was the common practice of fixing flagellate cells for light, confocal, or electron microscopy observations, which restricts the observed cells to a static state and prevents real-time observation of their behaviour or development. To overcome this limitation, a simple, cost-effective method has been developed to allow observation of living Trebouxia flagellate cells over several days [1]. With this method, the movement of the cells is spatially confined without compromising their viability, enabling the recording of images and videos. Compared to existing approaches, the new method allows detailed observation of cell development, motility, morphological differentiation, and interactions, including zoospore release and gamete fusion, providing a powerful tool to study asexual and sexual reproductive processes in Trebouxia and possibly other microalgae.
Materials and reagents
Biological materials
1. Trebouxia decolorans Ahmadjian [Culture Collection of the University of Trieste (Italy), originally isolated from Xanthoria parietina (L.) Th. Fr.]
2. T. gelatinosa Ahmadjian [Culture Collection of the University of Trieste (Italy), originally isolated from Flavoparmelia caperata (L.) Hale]
3. T. vagua Voytsekhovich & Beck [Culture Collection of the University of Trieste (Italy), originally isolated from Bagliettoa marmorea (Scop.) Gueidan & Cl. Roux]
4. T. angustilobata (Beck) Beck (SAG, strain number: 2204)
Note: All Trebouxia strains were cultivated on solid Trebouxia medium (TM, see Recipes) in Microbox Junior 40 vessels and subcultured every 3 weeks. Vessels were kept in a thermostatic chamber at 16 ± 1 °C and 23 ± 1 μmol photons·m-2·s-1 with a 12/12 h light/dark regime [1].
Reagents
1. ZnSO4·7H2O (Carlo Erba, catalog number: 494907)
2. MnCl2·4H2O (Honeywell Fluka, catalog number: 63543)
3. MoO3 (Sigma-Aldrich, catalog number: 267856)
4. CuSO4·5H2O (Sigma-Aldrich, catalog number: 939315)
5. Co(NO3)2·6H2O (Supelco, catalog number: 1.02536)
6. EDTANa2 (Sigma-Aldrich, catalog number: 27285)
7. KOH (Sigma-Aldrich, catalog number: 484016)
8. FeSO4·7H2O (Honeywell Fluka, catalog number: 12354)
9. H2SO4, 96% (Carlo Erba, catalog number: 410261)
10. NaNO3 (Carlo Erba, catalog number: 481757)
11. MgSO4·7H2O (Carl ROTH, catalog number: 8283.1)
12. NaCl (Applichem, catalog number: APA29425000k)
13. K2HPO4 (VWR, catalog number: 26930.260)
14. KH2PO4 (VWR, catalog number: 26936.260)
15. CaCl2·2H2O (Supelco, catalog number: 1.02382)
16. H3BO3 (Sigma-Aldrich, catalog number: B6768)
17. Casein yeast peptone (Sigma-Aldrich, catalog number: 39396)
18. D-(+)-glucose (Sigma-Aldrich, catalog number: G7021)
19. Agar (Sigma-Aldrich, catalog number: 05040)
Solutions
1. Trace elements stock solution (see Recipes)
2. EDTA stock solution (see Recipes)
3. Fe stock solution (see Recipes)
4. Bold’s basal medium (BBM) (see Recipes)
5. Trebouxia medium (TM) (see Recipes)
Recipes
The BBM is prepared starting from the stock solutions listed in the Reagent column of Recipe 4. For the trace elements, EDTA, and Fe solutions, detailed recipes are provided below (see Recipes 1–3). All the following stock solutions can be stored at 4 °C in glass bottles for at least 2–3 months after preparation. We recommend using autoclaved tips each time an aliquot is taken from the stock solutions and using different tips for each solution to avoid possible contamination and cross-contamination. If contamination is detected in a stock solution, discard it and prepare a fresh one before using it for the preparation of BBM or TM.
1. Trace elements stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| ZnSO4·7H2O | 8.82 g/L | 0.441 g |
| MnCl2·4H2O | 1.44 g/L | 0.072 g |
| MoO3 | 0.71 g/L | 0.0355 g |
| CuSO4·5H2O | 1.57 g/L | 0.0785 g |
| Co(NO3)2·6H2O | 0.49 g/L | 0.0245 g |
| dH2O | To a final volume of 50 mL |
Note: It may be necessary to autoclave the solution for all the reagents to dissolve.
2. EDTA stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| EDTANa2 | 50 g/L | 2.5 g |
| KOH | 31 g/L | 1.55 g |
| dH2O | To a final volume of 50 mL |
3. Fe stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| FeSO4·7H2O | 4.98 g/L | 0.249 g |
| H2SO4 (96%) | 1 mL/L | 0.05 mL |
| dH2O | To a final volume of 50 mL |
Caution: Always add acid to water and never water to acid!
4. Bold’s basal medium (BBM) [10]
| Reagent | Stock concentration | Volume (for 1 L) |
|---|---|---|
| NaNO3 | 25 g/L | 10 mL |
| MgSO4·7H2O | 7.5 g/L | 10 mL |
| NaCl | 2.5 g/L | 10 mL |
| K2HPO4 | 7.5 g/L | 10 mL |
| KH2PO4 | 17.5 g/L | 10 mL |
| CaCl2·2H2O | 2.5 g/L | 10 mL |
| H3BO3 | 11.4 g/L | 1 mL |
| Trace elements stock solution | - | 1 mL |
| EDTA stock solution | - | 1 mL |
| Fe stock solution | - | 1 mL |
| dH2O | To final volume of 936 mL |
Store in a glass bottle at 4 °C. It is better to have freshly prepared BBM every month, to be sure that all mineral nutrients are available to the algae when inoculation takes place and to minimise the possibility of contamination.
Prepare solid BBM according to Recipe 4 [10]. Transfer all solutions into a 1 L glass bottle and add 500 mL of dH2O. Place a magnetic stirring bar in the bottle and put it on a magnetic stirrer. Gradually add agar (15 g/L) while stirring and wait until it is evenly suspended from the bottom of the bottle before adding more. Note that it will only dissolve during autoclaving. Add dH2O to reach a final volume of 1 L.
5. Trebouxia medium (TM)
| Reagent | Final concentration | Quantity or volume (for 1 L) |
|---|---|---|
| NaNO3 | 0.5 g/L | 0.5 g |
| Casein yeast peptone | 10 g/L | 10 g |
| D-(+)-glucose | 20 g/L | 20 g |
| Agar | 15 g/L | 15 g |
| BBM | - | To final volume of 1 L |
Prepare TM according to Recipe 5 [11]. Pour 500 mL of liquid BBM into a 1 L glass bottle, add a magnetic stirring bar, and place it on a magnetic stirrer. Gradually add NaNO3 (0.5 g/L), casein yeast peptone (10 g/L), and D-(+)-glucose (20 g/L) to BBM while stirring until all reagents are completely dissolved. To prepare solid TM, gradually add agar (15 g/L) while stirring and wait until the agar is evenly suspended before adding more. Note that agar will only dissolve during autoclaving. Add BBM to reach a final volume of 1 L.
Note: Due to the presence of organic nutrients, TM is prone to contamination. Always prepare it fresh before use. This recipe is prepared according to the original formulation described in [11], which is based on Bold’s basal medium (BBM). TM is used only for maintenance of Trebouxia strains prior to the experiments; all microscopic observations are performed in BBM (see Recipe 4).
Laboratory supplies
1. 9-cm diameter Petri dishes (Sarstedt, catalog number: 82.1473.001)
2. 15 mL Falcon® tubes (Corning, Falcon®, catalog number: 352096)
3. 100% cellulose adsorbent paper (Aurora, model: Soft 850)
4. Magnetic stirring bar
5. Microbox Junior 40 vessels (Sac O2, model: O95/40+OD95)
6. Microscopy slides (Menzel-Gläser, model: 76 × 26 mm, ground edges)
7. Plastic plug obtained by cutting the base of a syringe needle (Terumo, catalog number: AN*2332R1)
8. Razor blades (Gillette, model: silver platinum plus)
9. Sterile disposable loops (VWR, catalog number: 612-9358)
10. Sterile disposable syringes (Terumo, catalog number: MDSS20ESE)
11. Steripack® pouches (PMS, catalog number: FP3050)
Equipment
1. 0.5–5 mL volume pipette (Eppendorf, catalog number: 3123000071)
2. 0.5–10 μL volume pipette (Eppendorf, catalog number: 3123000020)
3. 200 and 600 mL beakers (VWR, model: Borosilicate 3.3 glass line)
4. Analytical balance (Sartorius, model: Research)
5. Autoclave (Fedegari, model: FVS 1)
6. Biological hood (BIOAIR, model: TopSafe 1.5)
7. Light microscope (Zeiss, model: Primostar 3) equipped with camera (Zeiss, model: Axiocam 208 colour) and 40× objective (400× magnification)
8. Magnetic stirrer (ALC Apparecchi per Laboratori Chimici Srl, model: mivar)
9. Nylon net of 40 μm mesh (Spectrum Labs, Spectra/Mesh® Woven Filters)
10. Thermostatic chamber with adjustable temperature (15–20 °C) and dimmable, automated light control providing approximately 20 μmol photons·m-2·s-1 under a 12/12 h light/dark regime
11. Water distiller (Adrona, model: Crystal Ex)
Software and datasets
1. Axiocam 208 colour native software (Zeiss, version 1.3.8)
2. Fiji software (ImageJ, version 1.54f)
3. R software (v. 4.3.2; R Core Team, 2023)
Procedure
文章信息
稿件历史记录
提交日期: Oct 6, 2025
接收日期: Dec 7, 2025
在线发布日期: Dec 25, 2025
出版日期: Jan 20, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Boccato, E., Carniel, F. C. and Tretiach, M. (2026). A Simple Protocol for Periodic Live Cell Observation of Flagellate Stages in the Lichen Alga Trebouxia. Bio-protocol 16(2): e5566. DOI: 10.21769/BioProtoc.5566.
分类
植物科学 > 藻类学 > 蓝绿藻
细胞生物学 > 细胞分离和培养 > 细胞生长
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link