- EN - English
- CN - 中文
Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
利用基于分裂mNeonGreen的SAIYAN系统通过荧光信号检测内源性小GTP酶的激活
发布: 2026年01月05日第16卷第1期 DOI: 10.21769/BioProtoc.5557 浏览次数: 475
评审: David PaulAleksandra J. WierzbaShruthi Balachandra

相关实验方案
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2328 阅读
Abstract
Small GTPases function as molecular switches in cells, and their activation triggers diverse cellular responses depending on the GTPase type. Therefore, visualizing small GTPase activation in living cells is crucial because their activity is tightly regulated in space and time, and this spatiotemporal pattern of activation often determines their specific cellular functions. Various biosensors, such as relocation-based sensors and fluorescence resonance energy transfer (FRET)-based sensors, have been developed. However, these methods rely on interactions between activated GTPases and their downstream effectors, which limits their applicability for detecting activation of GTPases with unknown or atypical effectors. Recently, we developed a novel method utilizing split fluorescence technology to detect membrane recruitment of small GTPases upon activation, designated the Small GTPase ActIvitY ANalyzing (SAIYAN) system. This approach offers a new strategy for monitoring small GTPase activation based on membrane association and is potentially applicable to a wide range of small GTPases, including those with uncharacterized effectors.
Key features
• Visualizes the activation of small GTPases in living cells as mNeonGreen fluorescence signal.
• Can be applied to small GTPases whose effectors have not yet been identified.
• SAIYAN exploits the intrinsic property of small GTPases to associate with cellular membranes upon activation.
Keywords: Small GTPase (小GTP酶)Graphical overview
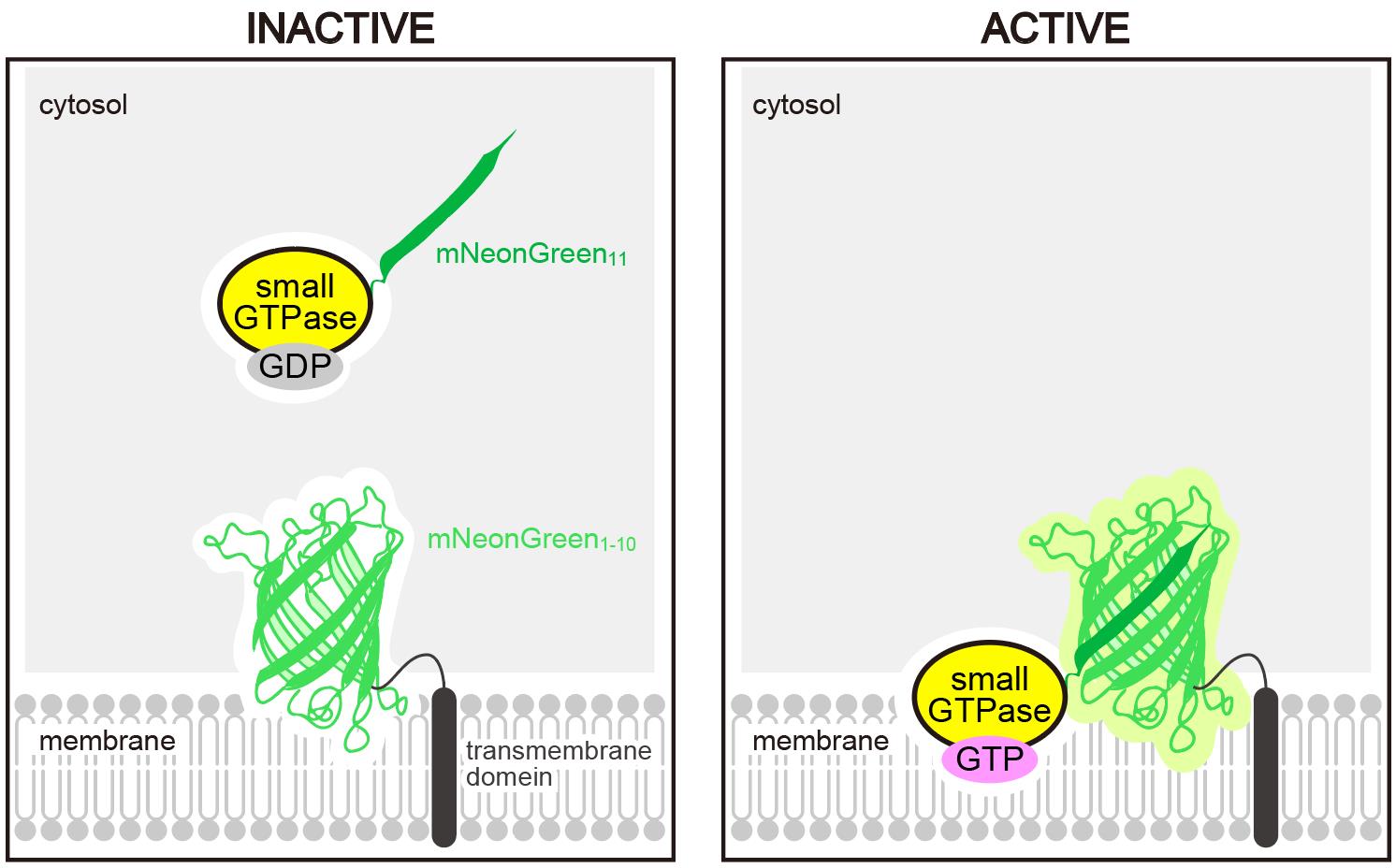
Schematic representation of the Small GTPase ActIvitY ANalyzing (SAIYAN) system. A short fragment of mNeonGreen (mNeonGreen11) is knocked into a small GTPase. Upon activation and membrane localization of the GTPase, mNeonGreen11 complements the remaining portion of mNeonGreen fused to a transmembrane domain, thereby reconstituting a functional fluorescent protein.
Background
Small GTPases function as molecular switches in a wide range of intracellular processes. The GDP-bound form is considered inactive, whereas guanine nucleotide exchange factors (GEFs) catalyze the exchange of GDP for GTP, converting the GTPase to its active form. Activated small GTPases interact with effector proteins to trigger various cellular responses. GTP hydrolysis, which is stimulated by GTPase-activating proteins (GAPs), then returns them to the inactive GDP-bound state. Thus, evaluating the activated form of small GTPases is of critical importance [1].
Several approaches have been developed to quantitatively assess the GTP-bound forms of small GTPases, such as assays using radioisotopes or pull-down techniques [2,3]. However, these methods require cell lysis and therefore cannot reveal the spatial distribution of activated small GTPases in living cells. The Raichu system enables detection of activated Ras using a fluorescence resonance energy transfer (FRET)-based biosensor, but this method requires overexpression of the GTPase itself as well as effector proteins that specifically bind to the GTP-bound state [4]. Relocation sensors, in which small GTPases are fused to fluorescent proteins, allow monitoring of their localization; however, because fluorescence is emitted from both GDP- and GTP-bound forms, background signals are often high [5].
Sar1 is a member of the Arf family of small GTPases that plays a pivotal role in COPII vesicle formation [6]. Upon activation by the GEF Sec12, Sar1 binds to the endoplasmic reticulum (ER) membrane. Membrane-bound Sar1 then recruits the Sec23/24 complex, forming the prebudding complex while concentrating cargo proteins destined for export. Together with Sec13/31, this leads to COPII vesicle budding from the ER membrane. Although Sec23 binds activated Sar1, it also functions as a GAP for Sar1, and thus, no conventional effector proteins for Sar1 exist. Consequently, the localization of activated Sar1 has remained elusive using traditional approaches [7].
We developed a new approach termed the SAIYAN system [8]. This method is based on a split mNeonGreen (mNG) strategy: a short fragment of mNeonGreen11 is fused to Sar1, while the complementary portion of mNG is expressed on the ER membrane. In this configuration, only activated Sar1, which binds to the ER membrane, can reconstitute the fluorescent mNG signal. This enables selective detection of the activated pool of Sar1, even in the absence of conventional effector proteins. Using this system, we revealed that activated Sar1 exhibits a distinctive distribution specifically in collagen-secreting cells [8]. Because this method allows visualization of GTPase activity in a manner dependent on activation state and subcellular localization, it can be broadly applied to other small GTPases whose localization is altered upon activation [9].
In this protocol, we provide a more detailed explanation of the methodology originally described in the previous publication, while also presenting ideas to generalize the approach for other small GTPases beyond Sar1. This overview clarifies the scope and purpose of the protocol and guides readers in adapting the method to different targets.
Materials and reagents
Biological materials
1. HEK293T cells (ATCC, catalog number: CRL-3216)
2. HeLa cells (ATCC, catalog number: CCL-2)
3. BJ-5ta cells (ATCC, catalog number: CRL-4001)
4. PrimeSTAR GXL DNA Polymerase (TAKARA, catalog number: R050A)
5. CUGA7 gRNA Synthesis kit (NIPPON Gene, catalog number: 314-08691)
6. DNeasy Blood & Tissue kit (QIAGEN, catalog number: 69504)
7. In-Fusion HD cloning kit (TAKARA, catalog number: 639649)
8. Guide-it Long ssDNA production system v2 (TAKARA, catalog number: 632666)
9. FastGene Gel/PCR Extraction kit (NIPPON GENE, catalog number: FG-91202)
10. Cas9 (TAKARA, catalog number: 632678)
11. Goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 488 (Thermo Fisher, catalog number: A-11034)
12. Goat anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 647 (Thermo Fisher, catalog number: A-21236)
13. pENTR/TRE vector (a gift from Dr. Kenji Kontani, Meiji Pharmaceutical University, Japan)
14. pSLIK-Neo vector (Addgene, catalog number: 25735)
15. pcDNA3.1(+) (Invitrogen, catalog number: V79020)
16. pRSV-Rev vector (Addgene, catalog number: 12253)
17. pMDLg/pRRE vector (Addgene, catalog number: 12251)
18. pMD2.G vector (Addgene, catalog number: 12259)
Reagents
1. D-MEM (SHIMADZU, catalog number: 05915)
2. Lipofectamine RNAi Max Transfection Reagent (Thermo Fisher, catalog number: 13378150)
3. DMEM (4.5 g/L Glucose) with L-Gln, sodium pyruvate, and 1.5 g/L sodium hydrogen carbonate, liquid (Nacalai, catalog number: 16971-55)
4. Medium 199 (Sigma, catalog number: M4530-500ML)
5. Fetal bovine serum (FBS) (Gibco, catalog number: 12662-029)
6. 0.5 g/L Trypsin/0.53 mM EDTA solution, with phenol red (Nacalai Tesque, catalog number: 32778-34)
7. Opti-MEM (Thermo Fisher, catalog number: 31985070)
8. Lipofectamine 2000 transfection reagent (Thermo Fisher, catalog number: 11668019)
9. Polyethylenimine, linear MW (25,000) (Polysciences, catalog number: 23966)
10. FuGENE 4K transfection reagent (Promega, catalog number: E5911)
11. GatewayTM LR ClonaseTM II enzyme mix (Thermo Fisher, catalog number: 11791020)
12. Polybrene solution (10 mg/mL) (Nacalai Tesque, catalog number: 12996-81)
13. Doxycycline hydrochloride n-hydrate (Fujifilm WAKO, catalog number: 049-31121)
14. Antibiotic G418 sulfate (Nacalai Tesque, catalog number: 08973-14)
15. NucleoSpin Gel and PCR Clean-Up kit (Macherey-Nagel, catalog number: 740609.50)
16. BSA (Sigma, catalog number: A7906)
17. NaN3 (Nacalai Tesque, catalog number: 31233-42)
18. PBS(-)
19. MilliQ
20. Anti-HA high affinity (3F10) (Roche, catalog number: 11867423001)
21. Goat anti-rat IgG (H+L) cross-adsorbed secondary antibody, Alexa FluorTM 568 (Thermo Fisher, catalog number: A-11077)
22. L-ascorbic acid phosphate magnesium salt n-hydrate (Fujifilm WAKO, catalog number: 013-12061)
23. Methanol (Nacalai Tesque, catalog number: 21915-93)
24. 4% paraformaldehyde (PFA) phosphate buffer solution (Nacalai Tesque, catalog number: 09154-56)
25. PermaFluor aqueous mounting medium (Epredia, catalog number: TA-030-FM)
Solutions
1. DMEM supplemented with 10% FBS (see Recipes)
2. Blocking buffer (see Recipes)
Recipes
1. DMEM supplemented with 10% FBS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| D-MEM | 500 mL | |
| FBS | 10% (v/v) | 50 mL |
2. Blocking buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BSA | 5% (w/v) | 25 g |
| NaN3 | 0.1% (w/v) | 0.5 g |
| PBS(-) 10× | 1× | 50 mL |
| MilliQ | top up to 500 mL |
Laboratory supplies
1. Adjustable micropipettes set, 0.1–1,000 μL
2. 50 mL tubes (Corning, catalog number: 430829)
3. 15 mL tubes (Corning, catalog number: 430791)
4. 1.5 mL tubes (BIOBIK, catalog number: ST-0150F)
5. 1.5 mL tubes (BIOBIK, catalog number: RC-0150)
6. 5 mL tubes (BIOBIK, catalog number: ST-500)
7. 5 mL pipettes (Greiner, catalog number: 606180)
8. 10 mL pipettes (Greiner, catalog number: 607180)
9. 25 mL pipettes (Greiner, catalog number: 760180)
10. 100 mm TC-treated culture dish (Corning, catalog number: 430167)
11. 60 mm TC-treated culture dish (Corning, catalog number: 430166)
12. Cell culture plate 6-well (WATSON, catalog number: 197-06CPS)
13. Cell culture plate 24-well (Corning, catalog number: 197-24CPS)
14. Tissue culture dish (surface: Cell+) (SARSTEDT, catalog number: 83.3902.300)
15. 0.22 µm syringe filter (gamma sterilized) (Membrane Solutions, catalog number: 61-9639-01)
16. 12 mm microscope cover glasses (thickness 0.13–0.16 mm) (Paul Marienfeld, catalog number: 0111520)
Equipment
1. Biosafety cabinet (PHCbi, catalog number: MHE-S1301A2)
2. Bioclean bench (PHCbi, catalog number: MCV-B131F)
3. CO2 incubator (PHCbi, catalog number: MCO-230AICUV)
4. Biomedical freezer (PHCbi, catalog number: MDF-MU539H)
5. Neon transfection system (Thermo Fisher, catalog number: MPK5000)
6. Vortex mixer (Scientific Industries, catalog number: SI-0286)
7. Centrifuge (KUBOTA, catalog number: S300T)
8. Cell sorter (BD Biosciences, model: BD FACS Melody)
9. Confocal microscope (Carl Zeiss, model: LSM900 with Airyscan2)
Software and datasets
1. ZEN Blue (Carl Zeiss, https://www.zeiss.com/microscopy/en/products/software/zeiss-zen.html) for microscopic image acquisition
2. ImageJ/Fiji for image analysis
3. Prism 10 for data analysis
Procedure
文章信息
稿件历史记录
提交日期: Sep 19, 2025
接收日期: Nov 23, 2025
在线发布日期: Dec 9, 2025
出版日期: Jan 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Maeda, M. and Saito, K. (2026). Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System. Bio-protocol 16(1): e5557. DOI: 10.21769/BioProtoc.5557.
- Maeda, M., Arakawa, M., Komatsu, Y. and Saito, K. 2024). mall GTPase ActIvitY ANalyzing (SAIYAN) system: A method to detect GTPase activation in living cells. J Cell Biol. 23(10): 202403179. https://doi.org/10.1083/jcb.202403179
分类
细胞生物学 > 细胞信号传导 > 胞内信号传导
细胞生物学 > 细胞成像 > 共聚焦显微镜
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link