- EN - English
- CN - 中文
Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera
基于高光谱相机的不同光照条件下叶绿体运动成像研究
(§ Technical contact) 发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5541 浏览次数: 694
评审: Pawan KumarAnonymous reviewer(s)
Abstract
Plants move chloroplasts in response to light, changing the optical properties of leaves. Low irradiance induces chloroplast accumulation, while high irradiance triggers chloroplast avoidance. Chloroplast movements may be monitored through changes in leaf transmittance and reflectance, typically in red light. We present a step-by-step procedure for the detection of chloroplast positioning using reflectance hyperspectral imaging in white light. We show how to employ machine learning methods to classify leaves according to the chloroplast positioning. The convolutional network is a method of choice for the analysis of the reflectance spectra, as it allows low levels of misclassification. As a complementary approach, we propose a vegetation index, called the Chloroplast Movement Index (CMI), which is sensitive to chloroplast positioning. Our method offers a high-throughput, contactless way of chloroplast movement detection.
Key features
• Protocol for detached leaves handled in laboratory conditions.
• Based on differential (dark-adapted versus irradiated) hyperspectral images of plant leaves.
• Data analysis includes machine learning methods and the calculation of a vegetation index.
• Requires irradiation equipment apart from the hyperspectral camera set.
Keywords: Arabidopsis thaliana (拟南芥)Graphical overview
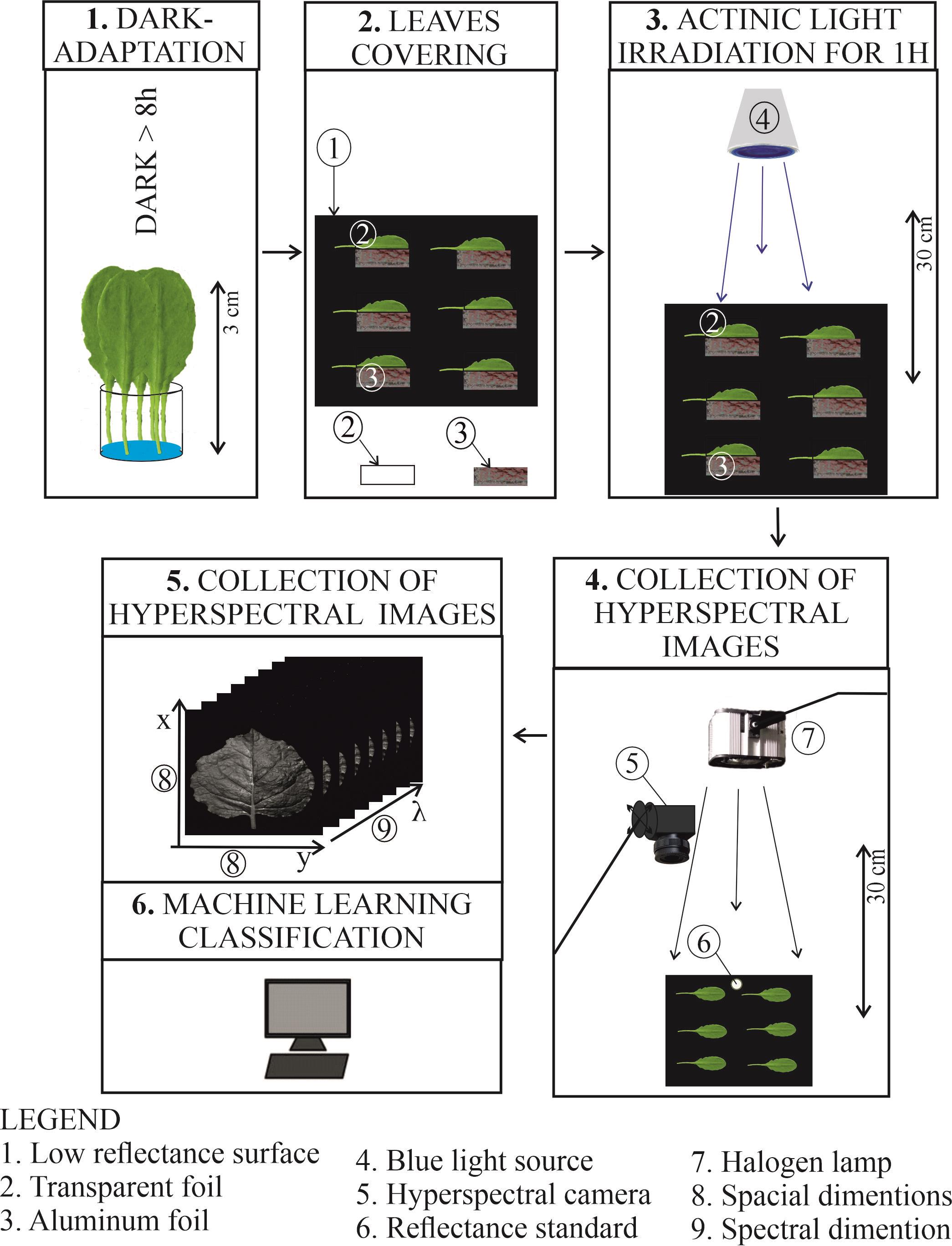
The main steps of the protocol for the detection of chloroplast responses based on leaf reflectance recorded with a hyperspectral camera. (A) Leaves or plants are dark-adapted overnight. (B) The next day, under a safe green light, half of the leaf is covered with aluminum foil and the other half with transparent foil on a Petri dish filled with black velvet. (C) The leaves are irradiated with blue light of high or low irradiance for 1 h to induce chloroplast movements. (D) Immediately after irradiation, the Petri dish is moved under the experimental setup, and a reflectance standard is added. The hyperspectral camera and a halogen light are simultaneously switched on. (E) The images are recorded on the computer and further processed. (F) The Chloroplast Movement Index may be calculated, or machine learning classification may be applied.
Background
Plants show distinct chloroplast positioning, dependent on light conditions. In low light, chloroplasts gather at cell walls lying perpendicular to the direction of incident light, a phenomenon known as the accumulation response. In high light, chloroplasts show avoidance response by moving to the cell walls parallel to the direction of incident light. Distinct dark positioning is also observed, with chloroplasts located at the bottom of the palisade cells [1].
Chloroplast movements have been examined using light microscopy for over a century [2,3]. Such observations are still important for assessing chloroplast responses, providing a detailed description of the movements of chloroplasts within the cell [4]. However, microscopic observations are laborious and usually restricted to the outer layers of the mesophyll in unfixed leaves. An alternative approach is to take advantage of the substantial changes in the optical properties of the whole leaf that result from chloroplast movements. Their optical effects are often visible to the naked eye. The slit assay is based on a simple observation that chloroplast avoidance makes leaves look pale, while chloroplast accumulation renders them darker [4]. Changes in leaf transmittance yield information on averaged chloroplast positions within the mesophyll cells and enable quantitative assessment of chloroplast movements. Photometric devices are used for measuring blue-light-induced changes in red light leaf transmittance [5–7]. They provide a continuous recording of the changes in leaf transmittance, which allows for studies of the kinetics of the process, though only one leaf can be examined at a time. Chloroplast movements may also be measured by changes in leaf reflectance [8–10]. Red light reflectance was used to monitor chloroplast movements with photosynthetic efficiency during the growth of Arabidopsis thaliana [11]. Two previous works [12,13] employed an integrating sphere to measure chloroplast movement-induced changes in diffuse reflectance. This approach involves the collection of whole (i.e., hemispherical) leaf reflectance, which is independent of the observation angle. This reduces the sensitivity of measurements to the leaf orientation and its surface undulations. However, such measurements are limited due to the requirement of direct contact with the leaf, which results in low throughput. Hyperspectral imaging is a fast, non-contact method of acquiring reflectance spectra in a spatially resolved manner; thus, it can be used to assess chloroplast positioning in several leaves at the same time. Such high throughput may be useful for phenotyping multiple mutants or studies on leaves collected in the field, in which a large sample is necessary due to the heterogeneity of the light conditions in natural environments. However, while a detector connected to an integrating sphere can measure light reflected to the whole hemisphere, the camera collects light reflected to a narrow cone. As a result, specular reflection from the leaf surface may reduce the accuracy of leaf classification according to chloroplast positioning, especially when leaves contain pronounced veins or their surface undulates. Specular reflectance may be reduced for such samples using light polarizers [10]. In an alternative approach, used in this protocol, classification of leaves is performed not based on raw reflectance, but using the complete visible light reflectance spectrum, through application of pretrained machine learning classifiers. The workflow of the protocol is shown in the Graphical overview.
Materials and reagents
Biological materials
1. Mature leaves from 6-week-old Nicotiana benthamiana
2. Fifth or sixth leaf from 4-week-old Arabidopsis thaliana, wild-type, ecotype Col-0, N6673 (NASC, www.arabidopsis.org)
Note: Plants are sown in Jiffy-7 pots (Jiffy Products International AS, Holand), vernalized at 4 °C for 2 days, and grown in a growth chamber (Sanyo MLR 350H, Japan) at 23 °C, 80% relative humidity, with a photoperiod of 10 h light and 14 h darkness, with irradiance of 70 µmol·m-2·s-1 supplied by fluorescent lamps (FL40SS.W/37, Sanyo, Japan). After 4 weeks, Nicotiana plants are transferred to pots with commercial soil and moved to a walk-in-type growth chamber supplied with white LEDs of 100 µmol·m-2·s-1 with a photoperiod of 10 h light and 14 h darkness.
Reagents
1. Tap water (400 ppm)
Laboratory supplies
1. Thick aluminum foil (0.03 mm) (Hutnik Sp. z o.o., Poland)
2. Transparent polyester foil (e.g., Alpha Autostat CT5, MacDermid, USA)
3. Paper towel, cellulose, two-layer (Merida, Poland)
4. Matte, black velvet (e.g., Musou Black VL Flock Sheet, Musou Black, Germany)
5. Petri dish, rectangular (100 mm × 100 mm × 20 mm) (Sarstedt, catalog number: 82.9923.422)
Equipment
1. Headwall nano hyperspectral camera (Nano-Hyperspec VNIR 400–1,000 nm, Headwall Photonics)
2. White diffuse reflectance standard (Spectralon or BaSO4-based reflectance standard, e.g., Edmund Optics, catalog number: #54-302)
3. 12 mm objective (Cinegon F/1.4/12-0906, C-Mount, 400–1,000 nm, Schneider Kreuznach)
4. Rotating platform (Sevenoak SK-ebh01 Pro, Andoer)
5. Manfrotto 143 magic arm with bracket (Videndum Media Solutions Spa)
6. Halogen lamp (Hedler HF 65, HEDLER Systemlicht GmbH)
7. Blue light LED panel (peak 455 nm, XLamp XQ-E HD Royal Blue Cree or equivalent blue light source)
Note: Several manufacturers offer royal blue LEDs that can be used as an alternative. However, LEDs described as PC-blue or PC-cyan are not suitable, as their spectrum features a prominent shoulder or second peak in the longer wavelength range.
8. Sensor (LI-COR Biosciences, model: LI-190R)
9. Leaf state analyzer (Walz, model: LSA-2050)
Software and datasets
1. Mathematica version 14 or later (Wolfram Research, USA); requires a license
2. All code has been deposited to GitHub: https://github.com/plantPhotobiologyLab/machine-learning-for-chloroplast-movement-detection (access date, 08/18/2025)
3. Original files with hyperspectral images of Nicotiana benthamiana and Arabidopsis thaliana (WT and phot2) leaves, including recordings shown in Figure 3 and Figure 4 of this protocol, can be downloaded from https://figshare.com/articles/dataset/Hyperspectral_images_of_Arabidopsis_thaliana_and_Nicotiana_benthamiana_leaves/30402409?file=58898569
4. Statistical analysis can be performed using the R language (version 4.5 available from https://cran.r-project.org/) in the RStudio integrated development environment (available from https://posit.co/products/open-source/rstudio/?sid=1)
Procedure
文章信息
稿件历史记录
提交日期: Aug 20, 2025
接收日期: Nov 6, 2025
在线发布日期: Nov 20, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Hermanowicz, P., Hebda, A. and Łabuz, J. (2025). Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera. Bio-protocol 15(24): e5541. DOI: 10.21769/BioProtoc.5541.
分类
植物科学 > 植物细胞生物学 > 细胞成像
植物科学 > 植物生理学
生物信息学与计算生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link