- EN - English
- CN - 中文
Station Holding During Rheotaxis: A Sensitive Assay of Lateral Line Function in Larval Zebrafish
基于趋流行为的定点停留:一种高灵敏度评估斑马鱼幼体侧线功能的行为学方法
发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5540 浏览次数: 712
评审: Alberto RissoneAnonymous reviewer(s)
Abstract
Hair cells are the sensory receptors of the auditory and vestibular systems in the inner ears of all vertebrates. Hair cells also serve to detect water flow in the lateral line system in amphibians and fish. The zebrafish lateral line serves as a well-established model for investigating hair cell development and function, including research on genetic mutations associated with deafness and environmental factors that cause hair cell damage. Rheotaxis, the ability to orient and swim in response to water flow, is a behavior mediated by multiple sensory modalities, including the lateral line organ. In this protocol, we describe a rheotaxis assay in which station holding behavior, which employs positive rheotaxis to maintain position in oncoming water flow, serves as a sensitive measure of lateral line function in larval zebrafish. This assay provides a valuable tool for researchers assessing the functional consequences of genetic or environmental disruptions of the lateral line system.
Key features
• Describes the method developed by Newton et al. [1] to assess lateral line function in larval zebrafish.
• Provides instructions on building the micro flume apparatus with updated information from the WashU Neurotech Hub.
• Uses DeepLabCut to track fish and SimBA to classify rheotaxis to compare lateral line–mediated behaviors in large cohorts of larval zebrafish.
Keywords: Rheotaxis (趋流行为)Background
Hair cells function as evolutionarily conserved sensory receptors within the auditory and vestibular systems of the vertebrate inner ear. These same cells act as sensory receptors for detecting water flow in the lateral line systems of amphibians and fishes [2–4]. In the lateral line system, bundles of hair cells, supporting cells, and innervating neurons located near the surface of the skin along the head and the body constitute functional units, called neuromasts, which detect hydrodynamic stimuli (changes in water velocity and turbulence) and sense low-frequency water flow. The hydrodynamic stimuli are transduced by hair cells from a mechanical stimulus into electrochemical signals. Hair cells release the excitatory transmitter glutamate onto afferent neurons of the lateral line ganglia, and the directional flow information is integrated in the hindbrain (lateral line nucleus and cerebellum), along with other sensory modalities interpretable by the central nervous system [3]. These features of sensory hair cell function are highly evolutionarily conserved, and their fundamental mechanisms for transducing sensory stimuli are similar across vertebrate species.
The zebrafish lateral line is a well-established model system for studying hair cell development and function, including studies evaluating the effects of mutations linked to deafness or environmental insults that damage hair cells [5–11]. In larval zebrafish, lateral line hair cells are located superficially along the skin, making them more accessible for experimentation than those of the mammalian ear, which are enclosed in the skull. This accessibility enables the use of various pharmacological treatments and imaging techniques, allowing researchers to apply reagents and visualize cellular processes in an intact larval fish. Yet despite the widespread experimental use of the zebrafish lateral line system in studies of hair cell sensory organ development, damage, and regeneration, there is currently no standard behavioral assay used to evaluate zebrafish lateral line function.
Rheotaxis—the ability to orient into and maintain position in water flow against oncoming current—is a behavior mediated by multiple sensory modalities, including visual, vestibular-tactile, chemotactile, and proprioceptive [12–14], and it includes the lateral line system. Rheotaxis helps fish maintain their position in the water flow and align their body upstream (i.e., positive rheotaxis [2,15]). A specific class of neuromasts, termed superficial neuromasts, is located on the surface of the body and has been shown to mediate rheotaxis [13]. Researchers have developed various methods to study rheotaxis in larval zebrafish, including measuring alignment in a rectangular flume [15], using computer vision to analyze swim patterns in radial flow [14], testing lateral line responses in a suction chamber [16], and employing a line actuator in a horizontal tube to reveal the role of the lateral line in detecting direction [17]. However, none of these approaches has been adopted for general use in laboratories.
Here, we describe a protocol and outline the essential components to reproduce our experimental rheotaxis assay, providing a validated behavior setup to study lateral line–mediated rheotaxis behavior in larval zebrafish. We sought to develop a behavioral assay and analytical methodology that is sensitive, spatiotemporally scalable, and robust enough to be adapted for use on a variety of species. The protocol incorporates computer vision and machine learning tools to precisely quantify the movement, velocity, and acceleration of the fish during rheotaxis. This assay provides the sensitivity needed to quantitatively assess the functional impact of both moderate and severe genetic or environmental disruptions of the lateral line system. For example, our assay has been used in prior studies to define lateral line–mediated behavior deficits in larval zebrafish with a mutation that impairs synaptic neurotransmission at the hair cell synapse [18] and in fish treated with both moderate and high doses of the ototoxic chemotherapy drug cisplatin [1,19].
Our behavioral setup consists of a translucent 3D-printed microflume with flow collimators providing consistent water flow that is delivered by an Arduino-controlled water pump to a small arena containing a single larval fish. The behavioral arena is illuminated with infrared light from below to eliminate visual guidance cues. The apparatus was developed in-house [1] and has subsequently been adapted and optimized at the Neurotech Hub at WashU (https://neurotechhub.wustl.edu/). We provide the method for printing and assembling the microflume and fish arena/basket (Figure 1) as well as for connecting the hardware and electronics (Figure 2). Furthermore, the flow rate can be parameterized and assayed using our flow velocity assay via visualization with methylene blue under bright light.
This protocol also describes how to process behavioral video recordings and track the fish’s body positions with DeepLabCut. DeepLabCut is a powerful standardized algorithm developed by Mathis et al. [20] that has been extensively used for behavioral tracking of a large variety of animal species, including zebrafish. It is a supervised deep learning tool based on residual networks (ResNet) for feature extraction that requires manual labeling of key body points of the fish, followed by training on a large image dataset (ImageNet), after which it generalizes to automatically track features in datasets of interest.
In addition, we describe the methodological use of SimBA [21], coupled with DeepLabCut, to algorithmically define, extract, and classify rheotaxis behavior. The extracted features are analyzed using customizable R scripts (provided in the GitHub companion to this protocol) to perform spatial, temporal, and spectral analysis, as well as measurements of the fish’s orientation (mean angle vector), velocity, and acceleration relative to the flow. Additional scripts generate heatmaps to visualize the average position occupied by the fish in the chamber arena over time. Time series analysis allows one to quantify both the distance travelled by the fish and the amplitude of oscillations of movements performed to maintain rheotaxis. Spectral analysis further reveals specific patterns of fluctuations in the swimming behavior of the fish to maintain rheotaxis under flow conditions (e.g., the overall trend and fluctuations in linear and angular movement.)
This protocol can be generalized to study other swimming behaviors in fish from different species by adapting the feature extraction script to another behavior of interest, like the C-start or more specific features like the tail posture, or changes in body movement and velocity. Furthermore, by integrating 3D tracking with multiple cameras and extending the size of the microflume, our protocol could be adapted for studying collective behavior, such as shoaling or schooling in multiple fishes.
Materials and reagents
Reagents
1. Methylene blue (Merck, catalog number: M4159-25G)
2. Distilled or nanofiltration water
3. NaCl (Sigma-Aldrich, catalog number: S9888)
4. KCl (Sigma-Aldrich, catalog number: P3911)
5. CaCl2·2H2O (Sigma-Aldrich, catalog number: C3306)
6. CaCl2 (Sigma-Aldrich, catalog number: C1016)
7. MgSO4·7H2O (Sigma-Aldrich, catalog number: 230391)
8. KH2PO4 (Sigma-Aldrich, catalog number: P0662)
9. Na2HPO4 (Sigma-Aldrich, catalog number: S3264)
10. NaHCO3 (Sigma-Aldrich, catalog number: S5761)
Solutions
1. 1× E3 medium (E3) (see Recipes)
2. 1× embryo medium (EM) (see Recipes)
Recipes
Note: Labs should use in the microflume whatever medium they typically use to maintain larval zebrafish. The following two recipes are routinely used in most zebrafish labs.
1. E3 medium (E3)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| E3 (60× stock) | 1× working solution | 1 L |
| NaCl | 5 mM NaCl | 34.4 g |
| KCl | 0.17 mM | 1.52 g |
| CaCl2·2H2O | 0.33 mM | 5.9 g |
| MgSO4·7H2O | 0.33 mM | 9.8 g |
| RO or DI H2O |
Dilute 16.7 mL of 60× E3 stock in 1 L of reverse osmosis (RO) or deionized (DI) H2O for 1× E3 working solution.
2. Embryo medium (EM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| EM (stock solution) | 1× working solution | 1 L |
| 1 M NaCl | 15 mM NaCl | 15 mL |
| 1 M KCl | 0.5 mM KCl | 500 μL |
| 1 M CaCl2 | 1 mM CaCl2 | 1 mL |
| 1 M MgSO4·7H2O | 1 mM MgSO4 | 1 mL |
| 1 M KH2PO4 | 0.15 mM KH2PO4 | 150 μL |
| 1 M Na2HPO4 | 0.042 mM Na2HPO4 | 42 μL |
| 1 M NaHCO3 | 0.714 mM NaHCO3 | 714 μL |
| RO or DI H2O | up to 1L |
Laboratory supplies
1. Pyrex© media bottle (Merck, catalog number: CLS13951L)
2. Polyethylene transfer pipette, 3 mL (to transfer larval fish) (VWR, catalog number: 16001-188)
3. Multi-well plate (e.g., Costar, catalog number: 3738)
4. Miniature ice packs (Millipore Sigma, catalog number: Z763195)
5. Partial immersion thermometer (e.g., Fisher Scientific, catalog number: 13-201-644) or temperature probe
6. Polyethylene transfer pipette, 1.5 mL, fine tip (to dispense methylene blue) (VWR, catalog number: 14673-010)
7. (Optional) Heat packs 2 × 5 inches with freezable gel (to keep larval fish warm during experiments)
Equipment
1. Microflume assembly
a. 3D printer (e.g., FormLabs Form 2, 3, or 4)
b. Clear resin (e.g., FormLabs Clear V5 resin)
c. Epoxy (e.g., Loctite Super Glue, liquid clear)
d. Clear silicone sealant (e.g., Loctite Silicone Sealant, clear)
e. Rubber gasket (3M 9448A silicone/rubber, 1.5 mm)
f. Polypropylene straws, 3 mm diameter (e.g., 5'' Sip Stirrer straws)
g. Thruster motor (Raboesch, catalog number: #108-01)
h. Acrylic top plate (TAP Plastics clear acrylic, 3 mm)
i. 88-micron nylon mesh for fish basket and netting in front of flume flow collimators (see Figure 1) (Small Parts Incorporated, CMN-88 A)
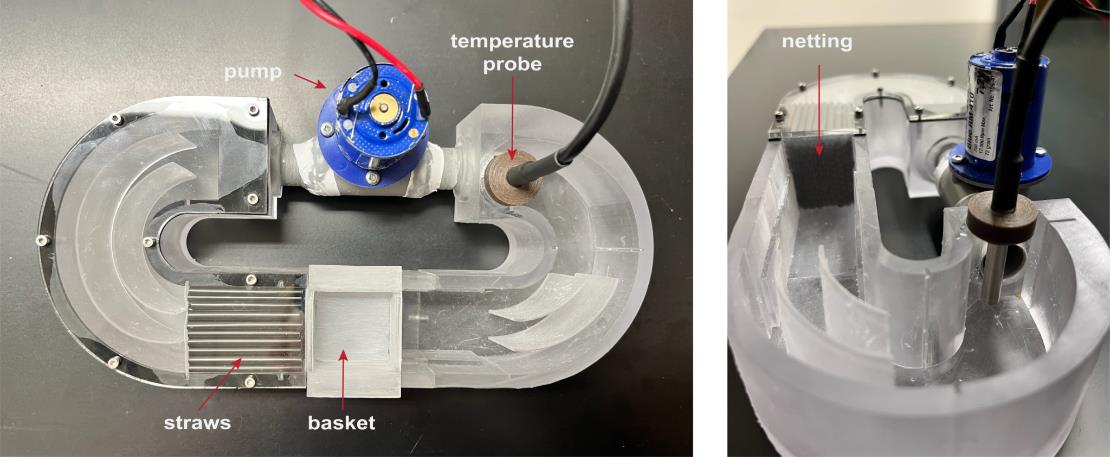
Figure 1. Updated microflume design. Photographs of the latest microflume configuration. (Left) Top-down view showing key components, including the pump/thruster motor (used to generate flow), a straw array (to promote laminar flow), the basket (to contain the zebrafish), and a temperature probe [used to monitor heat generated by the infrared (IR) light source]. In previous setups, a thermometer was manually placed in this location to track temperature. (Right) Side view of the microflume, highlighting the netting that secures the straw array and contributes to uniform flow distribution.
2. Arduino UNO R3, Osepp
The Arduino Uno is a microcontroller board based on the ATmega328P chip. It offers 14 digital I/O pins (6 with PWM capability), 6 analog inputs, a 16 MHz clock, USB connectivity, a power jack, an ICSP header, and a reset button. It enables users to build electronics projects by reading sensors, controlling actuators, and interfacing with various components through straightforward programming. Our setup uses 2 units: one to set the pulse width modulation (PWM) and the second with an LCD display to control the triggers for the camera and the pump. The Arduino code is available on the companion GitHub.
3. Digital display for Arduino, LCD display 16 × 2 Osepp
4. Inline rheostat (10k, 10-turn potentiometer with MC33926 Motor Driver Carrier) (Pololu item, catalog number: 1212)
5. Infrared (850 nm) light emitter (e.g., IR 130 LED IR ILLUMINATOR CMVision IR180-198 model: CM-IR130)
6. Diffusion barrier for IR light (Figure 2, component 4; three Kimwipes in a heat seal bag or light diffusing acrylic cut to the dimensions of the light source)
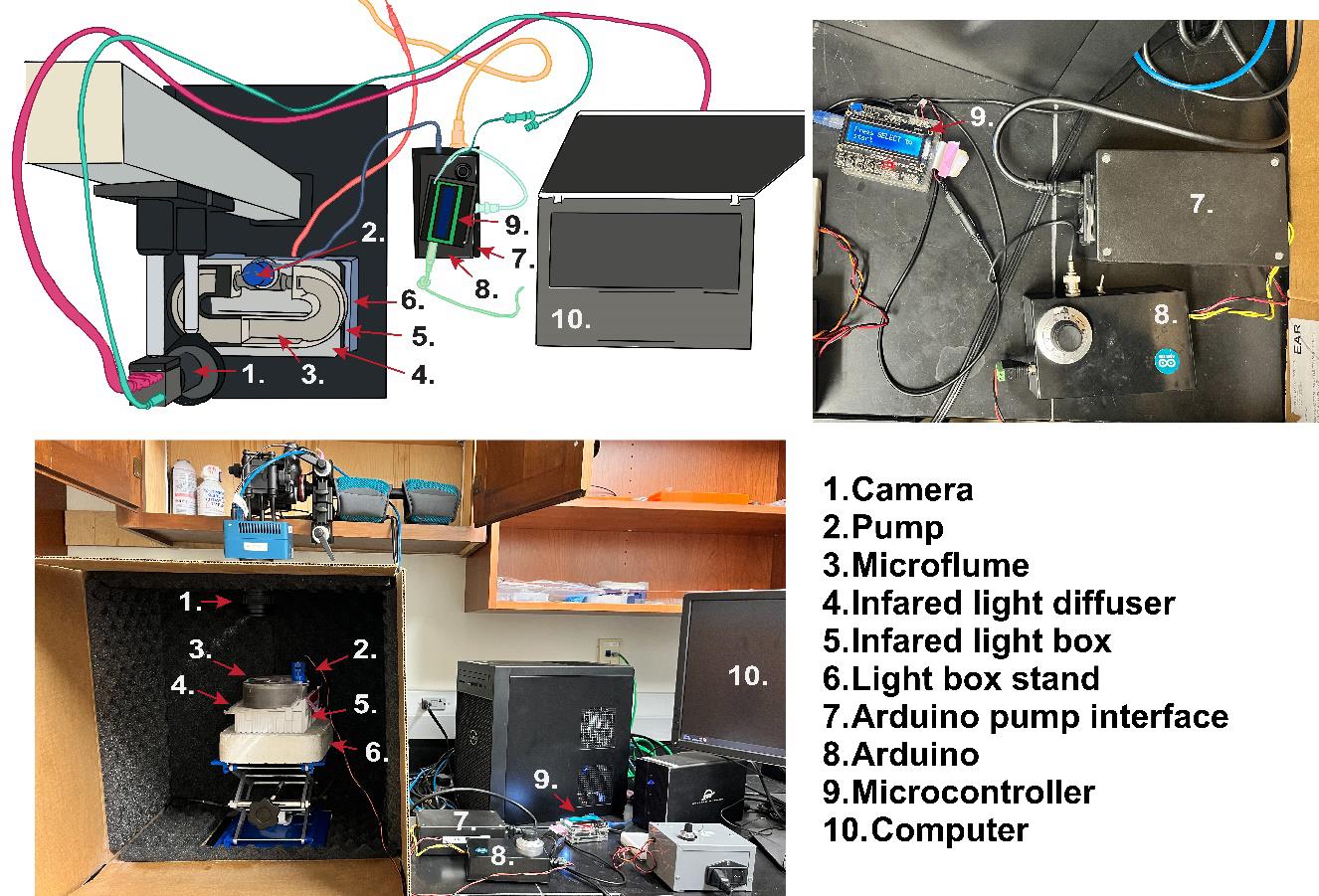
Figure 2. Rheotaxis rig setup. (Top-left) Example schematic diagram of the rheotaxis experimental setup at Amherst College, illustrating possible equipment layout and connections if using an optical rail with a camera and an infrared (IR)-filter and the platform for mounting a light source and the microflume. Components are labeled with numbers corresponding to the legend used consistently across all panels. Different components and their connections are color-coded for clarity: Magenta connection from camera to computer, teal connection from camera to Arduino Interface/Display, blue connection from micro flume pump to Arduino, coral connection from infrared lightbox to power strip, light green connection from Arduino controller to computer, aqua connection from Arduino to Arduino controller, and yellow connection from Arduino to power strip. (Top-right) Close-up view of the Arduino power supply (7), the Arduino and motor/driver, PWM (8), and the Arduino controller (9). (Bottom) Complete view of the assembled rheotaxis rig as implemented at WashU.
7. Blue light or broad-spectrum light emitter (for flow rate measurements with methylene blue)
8. Light-box stand and lift table (Figure 2, component 6)
a. WashU: a Styrofoam cooler lid (~8'' × 8'') cut to hold the IR emitter on top of an OESS Lift Table Aluminum Oxide Lab Stand Lifter Scientific Scissor Lifting Jack Platform 8'' × 8''
b. Amherst College: Custom 3D-printed light-box stand; optical rail for camera adjustment instead of moving the light-box stand.
9. Camera
a. WashU: high-speed camera (SC1 without IR filter, Edgertronic.com) with a 60 mm manual focus macro lens (Nikon) and 64 GB SD cards
b. Amherst College: Ximea MQ013MG-ON
10. A small screw (3–5 mm long) to substitute for a larval fish when focusing the camera
11. Tripod or optical rail for camera (Amherst College: Thorlabs XT66 66 mm Optical Construction Rail)
12. Computer
a. WashU: Windows 10 operating system based on a Dell Precision 3630 workstation with an Intel Xeon E-2246G processor, 64 GB RAM, multiple 2TB SSD hard drives, EVGA GFORCE RTX 2080Ti video card (GPU), dual 24'' 4 K monitors, Dell Thunderbolt 3 PCIe Card, OWC Mercury Elite Pro Dock (TB3RSDK24T), 24TB Thunderbolt 3 Dock and Dual-Drive RAID configured as 12TB RAID 1
b. Amherst College: ThinkPad T14 laptop computer running Windows 10 (64-bit) with 14-inch UHD (1,920 × 1,080) display and an i5 quad-core processor with 16 GB memory and a 512 GB SSD hard drive
13. Optional: Small LED flashlight (for use during setup of experimental runs in the dark)
14. Optional: Benchtop incubator next to the behavior setup (for housing larval fish during experiment) [WashU: MyTempMini (Benchmark)]
Software and datasets
1. DeepLabCut2.2b8 (maDLC43,44), Python (3.6 or later), and necessary dependencies (https://github.com/DeepLabCut/DeepLabCut/blob/master/docs/installation.md).
2. SimBA SimBAxTF-development version 68, Python, Git, FFmpeg, and all dependencies (https://github.com/sgoldenlab/simba/blob/master/docs/installation.md).
Procedure
文章信息
稿件历史记录
提交日期: Sep 25, 2025
接收日期: Nov 10, 2025
在线发布日期: Nov 20, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Cohen-Bodénès, S., Malak, E. I., Trapani, J. G., Gaidica, M., Militchin, V. A., Newton, K. C. and Sheets, L. (2025). Station Holding During Rheotaxis: A Sensitive Assay of Lateral Line Function in Larval Zebrafish. Bio-protocol 15(24): e5540. DOI: 10.21769/BioProtoc.5540.
分类
神经科学 > 行为神经科学 > 感觉运动反应
神经科学 > 行为神经科学 > 实验动物模型
生物信息学与计算生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link