- EN - English
- CN - 中文
High Precision Antibody-Free Microtubule Labeling for Expansion Microscopy
用于膨胀显微成像的高精度无抗体微管标记方法
发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5539 浏览次数: 1263
评审: Elena A. OstrakhovitchAnonymous reviewer(s)
Abstract
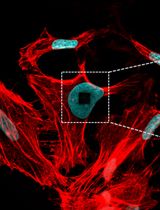
Expansion microscopy (ExM) enables nanoscale imaging of biological structures using standard fluorescence microscopes. Accurate labeling of cytoskeletal filaments, such as microtubules, remains challenging due to structural distortion and labeling inaccuracy during sample preparation. This protocol describes an optimized method combining detergent extraction and NHS-ester labeling for high-precision visualization of microtubules in expanded samples. Cytoplasmic components and membranes are selectively removed, preserving the ultrastructure of the microtubule network. Microtubules are digested into peptides during expansion and subsequently labeled at their N-termini using NHS-ester dyes, eliminating the need for antibodies. Effective fluorophore displacement of ~1 nm or lower is achieved, depending on the applied expansion factor. The protocol is compatible with both in vitro and cellular samples and can be integrated into a wide range of ExM workflows. Labeled microtubules can serve as internal reference standards for correcting expansion factors in ExM datasets.
Key features
• Employs detergent extraction with accessible commercial reagents to isolate cytoskeletal structures and reduce background from membranes and cytoplasmic proteins in fluorescence microscopy.
• Avoiding excessive aldehyde fixation preserves amines required for gel polymer integration while NHS-ester labeling of tubulin amines reduces linkage error, enabling accurate molecular localization.
• Compatible with post-expansion workflows; labels newly generated peptide N-termini after digestion, enabling high-resolution fluorescence imaging with minimal linkage error and high signal-to-noise ratio (SNR).
• Suitable for integration into diverse ExM protocols and useful as a reference standard for expansion factor correction.
Keywords: Detergent-extraction (去垢剂抽提)Graphical overview
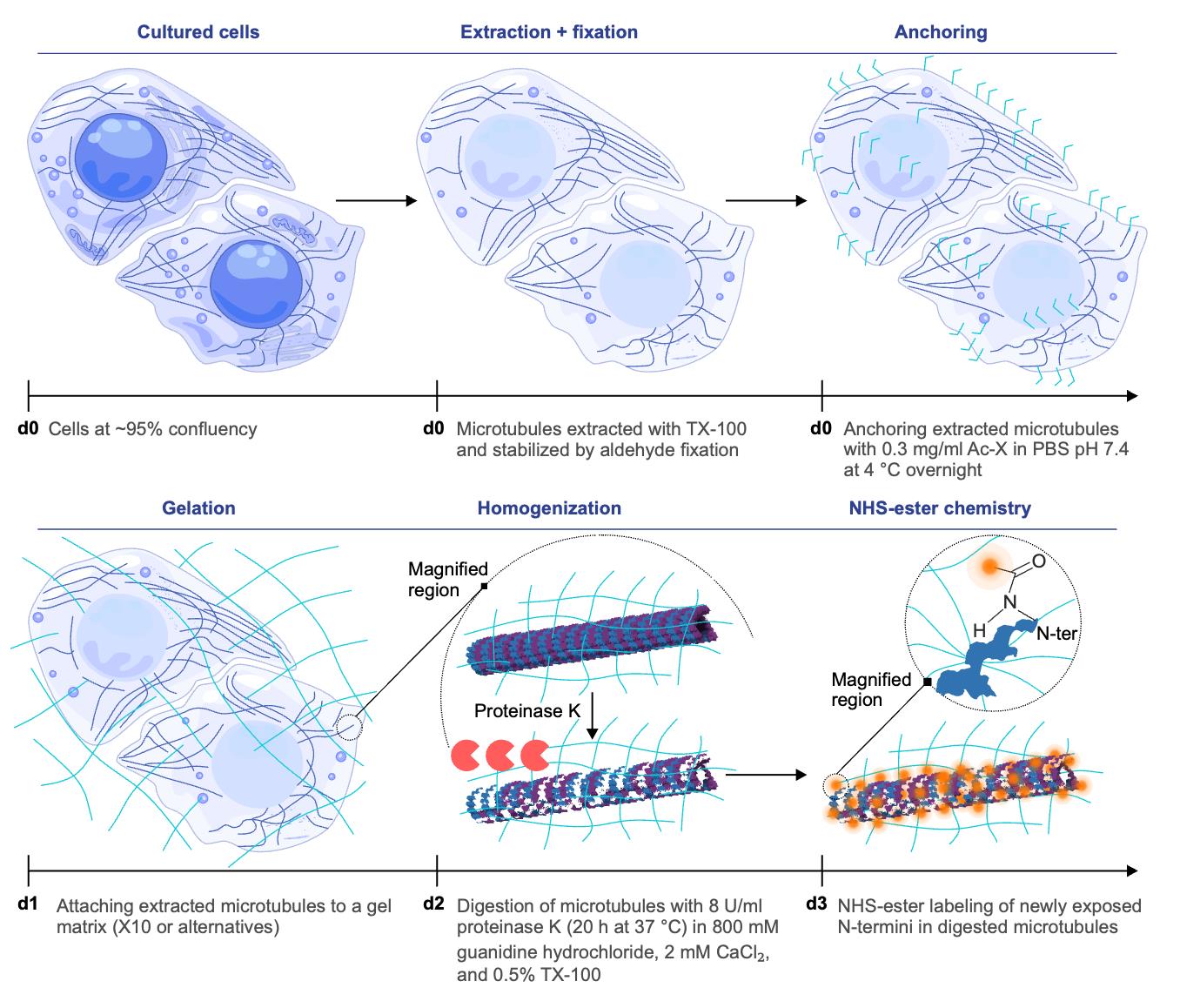
Antibody-free strategy for homogeneous expansion and direct microtubule labeling. In this schematic, “d” followed by a number indicates the day on which each step is performed. Microtubules are extracted and chemically stabilized, then covalently anchored to the expandable gel matrix. During the homogenization step on d2, a magnified region is shown to illustrate the process at the single microtubule level, representative of the overall sample. Proteolysis ensures uniform softening of the specimen and exposes new protein N-termini, which can be directly tagged by NHS-esters. The samples are subsequently expanded by water infusion, which swells the gel isotropically. This approach minimizes linkage error, since the effective contribution of the dye size (~1 nm) becomes negligible when scaled down by the expansion factor. The combination of homogeneous anchoring, complete digestion, direct chemical labeling after partial expansion, and isotropic expansion enables dense, antibody-free visualization of microtubules at nanoscale resolution.
Background
The advent of super-resolution microscopy has transformed cell biology by overcoming the diffraction limit and enabling visualization of subcellular structures with nanometer-scale precision [1–3]. Expansion microscopy (ExM [4]) achieves this not through complex optics but by physically enlarging specimens embedded in swellable hydrogels. The final resolution depends on the achieved expansion factor (e.g., 4× [4,5], 10× [6], 20× [7], or higher with gel iteration protocols [8–10]), which is determined by the composition of the hydrogel and the extent of protein homogenization [4–10]. Accurate calibration of the expansion factors requires internal reference structures with well-defined geometry and consistent labeling, which remains a significant challenge. For a considerable time, the nuclear pore complex (NPC) was used as a reference structure in both super-resolution [11,12] and expansion microscopy [9,13–15], owing to its well-defined geometry. Yet, with the advancement of ExM protocols and their integration with super-resolution methods, NPCs became less practical for this role. Their relatively large diameter means that they no longer provide a stringent measure of the effective resolution or expansion factor achievable today. We therefore turned to a smaller and more uniform intracellular structure: the microtubule network [15]. Microtubules are particularly well suited for this purpose because their cylindrical architecture, assembled from regularly arranged α/β-tubulin polymers with an outer diameter of about 25 nm, is highly predictable and conserved [16,17]. Their widespread presence in cells and uniform shape essentially make them a good choice for nanoscale rulers [15,18]. However, imaging microtubules in cells typically relies on antibody-based labeling [19], which introduces a spatial displacement of 30–80 nm between the fluorophore and the tubulin backbone due to the physical size and orientation of the antibody layers [15,19]. Nanobodies can reduce this displacement because of their smaller size, although inconsistencies in epitope accessibility still introduce variability.
To address this limitation, we developed a protocol that combines detergent extraction and NHS-ester labeling, bypassing the need for immunostaining. Cells are treated with Triton X-100 to remove membranes and soluble cytoplasmic proteins, preserving the cytoskeletal framework. Fluorescent NHS-esters are then used to label accessible amine groups, allowing direct tagging of the protein backbone with a displacement of approximately 1 nm or lower, depending on the expansion factor. Importantly, labeling targets not only lysine side chains and N-termini of intact proteins but also newly exposed amines generated during the digestion process. This significantly increases the density of labeling and enhances the signal-to-noise ratio. Microtubules, being the most prominent retained filaments, are readily distinguished from smaller cytoskeletal structures such as actin filaments. Their diameters are approximately 2–3 times larger than those of other cytoskeletal components, making them particularly amenable to visualization using our high-precision expansion protocol. This methodological advantage is consistent with previous findings [20], which effectively visualized and spatially resolved microtubules, intermediate filaments, and actin filaments within the same fixed cells. The digestion buffer contained 2 mM Ca2+, which may induce minor perturbations in actin organization. However, because the cells were chemically fixed prior to digestion, the overall integrity of the actin cytoskeleton is expected to remain largely preserved. The protocol has been validated using high-resolution techniques, including one-step nanoscale expansion (ONE) microscopy and expansion stimulated emission depletion (ExSTED) [15], with achieved resolutions of under 5 nm and approximately 8 nm, respectively. It is compatible with a variety of ExM workflows, including standard 4× [4] and 10× [6] protocols as well as U-ExM [5]. By eliminating antibody-induced displacement and providing a reliable cytoskeletal reference, this method improves the accuracy of structural analyses in ExM, including the study of microtubule-associated proteins and post-translational modifications in their native context.
Critical steps of the protocol
This method builds on an established cytoskeleton isolation protocol [21], incorporating key optimizations for ExM compatibility: mild fixation (0.5%–1% glutaraldehyde), controlled extraction using 2% Triton X-100 with Taxol, and post-expansion NHS-ester labeling. Each of these steps is outlined and discussed in detail in Table 1.
Table 1. Critical concepts for reliable microtubule expansion and visualization
| Critical step | Description |
| Mild fixation | Uses low glutaraldehyde concentrations to preserve microtubule architecture while avoiding excessive cross-linking. This balance ensures efficient gel anchoring and prevents artifacts from rigid or incompletely digested structures, which in turn can lead to non-homogeneous expansion factors. |
| Controlled extraction | Employs 2% Triton X-100 with Taxol to solubilize membranes without depolymerizing microtubules. This maintains cytoskeletal integrity and ensures that filaments remain accessible for anchoring and subsequent expansion. |
| Post-expansion labeling | Applies NHS-ester dyes after homogenization, when peptides are uniformly accessible. This minimizes labeling bias, reduces linkage error compared to antibodies, and produces dense, isotropic signal along microtubules. Fluorescein is suited for confocal imaging or ONE microscopy, whereas STAR635P enables successful STED imaging on expanded specimens. Other NHS-ester dyes can be used, but hydrophobic dyes should be avoided as they aggregate on negatively charged peptides and generate nonspecific bright signal. |
Materials and reagents
Cell line
1. HeLa cells were used in this protocol for microtubule extraction and subsequent labeling using NHS-ester chemistry
Reagents
1. Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, catalog number: 41965039)
2. Trypsin-EDTA (0.25%), phenol red (Gibco, catalog number: 25200072)
3. Fetal bovine serum (FBS) (Gibco, catalog number: 10270106)
4. Penicillin-streptomycin (Thermo Fisher Scientific, catalog number: 15140148)
5. Dulbecco’s phosphate-buffered saline (PBS) (1×) (Gibco, catalog number: 14190169)
6. Piperazine-(N,N’-bis(2-ethanesulfonic acid) (PIPES) sodium salt (Merck, catalog number: P2949)
7. Tris(hydroxymethyl)aminomethane (Tris) base (Merck, catalog number: 252859)
8. Magnesium chloride (MgCl2) (Merck, catalog number: 208337)
9. Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) (Merck, catalog number: E3889)
10. Triton X-100 (Merck, catalog number: T8787)
11. Polyethylene glycol (PEG) (MW 20,000) (Merck, catalog number: 86101)
12. Phalloidin (Thermo Fisher Scientific, catalog number: P3457)
13. Taxol (paclitaxel) (Merck, catalog number: T7402)
14. Electron microscopy–grade glutaraldehyde solution (50%) (Merck, catalog number: G7651)
15. Acryloyl-X SE (succinimidyl ester) (Thermo Fisher Scientific, catalog number: A20770)
16. Fluorescein NHS ester (Thermo Fisher Scientific, catalog number: 46409)
17. Anhydrous dimethyl sulfoxide (DMSO) (Merck, catalog number: 276855)
18. Sodium bicarbonate (Merck, catalog number: S6014)
19. Guanidine hydrochloride (Merck, catalog number: G3272)
20. Calcium chloride (CaCl2) (Merck, catalog number: C4901)
21. Proteinase K (Merck, catalog number: P4850)
Solutions
1. PIPES stock solution (see Recipes)
2. MgCl2 stock solution (see Recipes)
3. CaCl2 stock solution (see Recipes)
4. EGTA stock solution (see Recipes)
5. PEM buffer solution (see Recipes)
6. Taxol stock solution (see Recipes)
7. Phalloidin stock solution (see Recipes)
8. Extraction solution (see Recipes)
9. Fixation solution (see Recipes)
10. Acryloyl-X SE stock solution (see Recipes)
11. Digestion buffer (see Recipes)
12. Digestion solution (see Recipes)
13. Sodium bicarbonate buffer solution (see Recipes)
14. Fluorescein NHS ester stock solution (0.2 g/mL) (see Recipes)
Recipes
1. PIPES stock solution (200 mM in 500 mL)
Dissolve 32.43 g of PIPES sodium salt in 450 mL of ddH2O, add 1 M KOH until it dissolves, add ddH2O to make up the volume to 500 mL, and adjust the pH to 6.9. Store at 4 °C for short-term and -20 °C for long-term storage.
2. MgCl2 stock solution (10 mM in 500 mL)
Dissolve 0.476 g of MgCl2 powder in 500 mL of ddH2O. Store at 4 °C for short-term and -20 °C for long-term storage.
3. CaCl2 stock solution (500 mM in 20 mL)
Dissolve 1.1 g of CaCl2 powder in 20 mL of ddH2O. Store in a 50 mL centrifuge tube at 4 °C.
4. EGTA stock solution (10 mM in 200 mL)
Dissolve 0.76 g of powder in 200 mL of ddH2O. Store at 4 °C for short-term and -20 °C for long-term storage.
5. PEM buffer solution (100 mM in 500 mL)
Mix 250 mL of PIPES with 50 mL of MgCl2 stock and 50 mL of EGTA stock. Adjust the remaining 150 mL volume with ddH2O. Check the pH and adjust it by gradual addition of 1 M KOH using a micropipette while stirring continuously until the solution reaches the target pH of 6.9. Final composition of the PEM buffer becomes [PIPES] = 100 mM, [MgCl2] = 1 mM, and [EGTA] = 1 mM. This solution can be stored at room temperature (RT).
6. Taxol stock solution (1 mM)
Dissolve 1 mg of Taxol powder in 1.17 mL of anhydrous DMSO and store it at -20 °C. It is recommended to make small aliquots of 10 μL for single use and not to reuse the same aliquot.
7. Phalloidin stock solution (1 mM)
Dissolve 1 mg of phalloidin powder in 1.25 mL of anhydrous DMSO to get a 1 mM stock solution of phalloidin. It should be stored at -20 °C and may be reused.
8. Extraction solution (50 mL)
| Reagent | Final concentration | Volume/weight |
| Triton X-100 | 2% (v/v) | 1 mL |
| PEG | 4% (w/v) | 2 g |
| Taxol stock solution (1 mM) | 2 μM | 100 μL |
| Phalloidin stock solution (1 mM) | 2 μM | 100 μL |
| PEM buffer | ~100 mM | 48.8 mL |
Dissolve 2 g of PEG 20000, 1 mL of Triton X-100, 100 μL of Taxol stock solution (1 mM), and 100 μL of phalloidin stock solution (1 mM) in 48.8 mL of PEM buffer. Phalloidin is included in the extraction solution to stabilize filamentous actin (F-actin) and prevent its depolymerization during detergent extraction. It binds specifically and with very high affinity to only polymerized F-actin, thereby preserving existing actin filaments without influencing microtubule integrity. This selective binding stabilizes actin structures against chemical or mechanical disruption while allowing simultaneous maintenance of microtubules, which are independently stabilized by Taxol. Thus, the inclusion of phalloidin helps retain the native architecture of both filament systems for accurate structural analysis.
9. Fixation solution
Dilute the EM-grade glutaraldehyde to 0.5% in 100 mM PEM buffer (to pH 7.3). Glutaraldehyde is chemically unstable in aqueous solution and tends to undergo spontaneous polymerization and oxidation over time, especially near neutral pH. These reactions reduce the proportion of active monomeric aldehyde groups responsible for effective protein cross-linking and compromise fixation efficiency. Consequently, freshly diluted EM-grade glutaraldehyde ensures maximal reactive aldehyde availability and reproducible fixation of cellular structures. EM fixation guidelines recommend preparing glutaraldehyde solutions immediately before use to maintain optimal fixative performance. Hence, we recommend using a freshly prepared solution.
10. Acryloyl-X SE stock solution (10 mg/mL)
Dissolve 5 mg of material in 500 μL of anhydrous DMSO to get a 10 mg/mL stock solution and store it at -20 °C.
11. Digestion buffer (100 mL)
| Reagent | Final concentration | Volume/weight |
| Tris base | 100 mM | 1.2 g |
| CaCl2 stock solution (500 mM) | 2 mM | 400 μL |
| Triton X-100 | 0.5% | 500 μL |
| Guanidine hydrochloride | 800 mM | 7.6 g |
| ddH2O | N/A | 99 mL |
Dissolve 1.2 g of Tris base in 99 mL of ddH2O, add 400 μL of 500 mM CaCl2 stock solution to it, and adjust the pH to 8. Then, add 500 μL of Triton X-100 and 7.6 g of guanidine hydrochloride to it and mix using a magnetic stirrer until a clear solution is obtained. Remember not to add Triton X-100 and guanidine hydrochloride before adjusting the pH as they can damage the electrode. The solution can be stored at -20 °C for 6 months. We do not recommend freeze-thawing this solution multiple times; hence, it is better to make 1.5 mL aliquots in microcentrifuge tubes.
12. Digestion solution
The digestion buffer was thawed from frozen stocks and incubated at 37 °C for 30 min prior to use. Proteinase K (800 U/mL) was diluted 1:100 in the buffer, yielding a working concentration of 8 U/mL. For sample processing, 2 mL of the digestion solution was used per well of a 6-well plate, or 600 μL per well of a 12-well plate, ensuring complete immersion of the gels.
13. Sodium bicarbonate buffer solution (100 mM at pH 8)
Dissolve 4.2 g of sodium bicarbonate in 500 mL of ddH2O, adjust the pH to 8.0, and store the solution at RT.
14. Fluorescein NHS ester stock solution (0.2 g/mL)
Dissolve 1 g in 5 mL of anhydrous DMSO to obtain a 200 mg/mL solution. Preserving DMSO in an anhydrous state is challenging as it is highly hygroscopic and readily absorbs moisture from the atmosphere, which can compromise its anhydrous nature and affect solubility for moisture-sensitive reagents such as NHS esters. Therefore, DMSO should be stored in a tightly sealed amber glass bottle under dry conditions at RT (15–30 °C) and protected from light to prevent moisture uptake. NHS-ester dyes are inherently sensitive to hydrolysis, particularly in the presence of moisture. Once dissolved in anhydrous DMSO, their stability decreases markedly, and such stock solutions should be used within three months to prevent hydrolysis and loss of coupling efficiency. We recommend preparing small aliquots of 50–100 μL (for single use) and storing them at -20 °C.
Laboratory supplies
1. 12-well cell culture plate (Thermo Fisher Scientific, catalog number: 150200)
2. 6-well cell culture plate (Thermo Fisher Scientific, catalog number: 140675)
4. 25 cm2 tissue culture flask (Thermo Fisher Scientific, catalog number: 156367)
5. Microscope slides (Epredia, catalog number: J1800AMNZ)
6. 22 × 40 mm #1.5 coverslip (Electron Microscopy Sciences, catalog number: 72204-03)
7. 60 mm Petri dish (Fisher Scientific, catalog number: FB0875713A)
8. Expansion chamber (Corning, catalog number: 431111)
9. Polystyrene weighing tray (Merck, catalog number: HS1421C)
Equipment
1. Cell culture incubator (Labotec, model: Incubator C200)
2. Double-distilled water dispenser (Sartorius, model: Arium® Pro)
3. -80 °C freezer
4. -20 °C freezer
5. Refrigerator (4 °C)
6. Orbital shaker (Heidolph, model: Rotamax 120)
7. Phase contrast microscope (Zeiss, model: Axiovert 25)
8. Fluorescence microscope (Olympus, model: IX71)
9. STED microscope (Leica Microsystems, model: SP5)
10. 3D STED microscope (Leica Microsystems, model: Stellaris 8)
11. Abbe-refractometer (Krüss Optronic GmbH, Germany, model: AR4)
Software and datasets
1. ImageJ version 1.53j was used. ONE plugin is compatible with newer versions.
2. CorelDraw
Procedure
文章信息
稿件历史记录
提交日期: Sep 15, 2025
接收日期: Nov 6, 2025
在线发布日期: Nov 19, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Chowdhury, R., Krah, D., Ntolkeras, A., Heimbrodt, A. and Shaib, A. H. (2025). High Precision Antibody-Free Microtubule Labeling for Expansion Microscopy. Bio-protocol 15(24): e5539. DOI: 10.21769/BioProtoc.5539.
分类
细胞生物学 > 细胞结构 > 微管
细胞生物学 > 细胞成像 > 超分辨率成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link