- EN - English
- CN - 中文
Improved Immunohistochemistry of Mouse Eye Sections Using Davidson's Fixative and Melanin Bleaching
采用 Davidson 固定液和黑色素漂白法优化小鼠眼组织切片的免疫组化染色
发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5510 浏览次数: 1562
评审: Pilar Villacampa AlcubierreAnonymous reviewer(s)
Abstract
Immunohistochemistry (IHC) and immunofluorescence (IF) are fundamental molecular biology techniques to assess protein expression. However, the melanin present normally in the eye in the uveal tract (choroid, iris, and ciliary body) and the retinal pigment epithelium (RPE) poses a significant challenge for IHC and IF. This is because melanin interferes with both chromogenic and fluorescent detection methods. Additionally, formalin fixation, which is commonly used for IHC, can result in shrinkage and loss of cellular detail in the eye. This protocol provides an optimized approach using Davidson’s fixative with a hydrogen peroxide bleaching step to eliminate melanin interference in the mouse eye, improving the quality and interpretability of IHC analyses of the uveal tract and RPE. It is particularly useful for the analysis of uveal melanoma.
Key features
• Davidson’s fixative minimizes shrinkage of ocular tissues and preserves cell structure better than formalin.
• Hydrogen peroxide bleaching step removes all melanin, even in highly pigmented samples.
• The protocol is compatible with other tissues that require melanin removal.
Keywords: Immunohistochemistry (免疫组化)Graphical overview
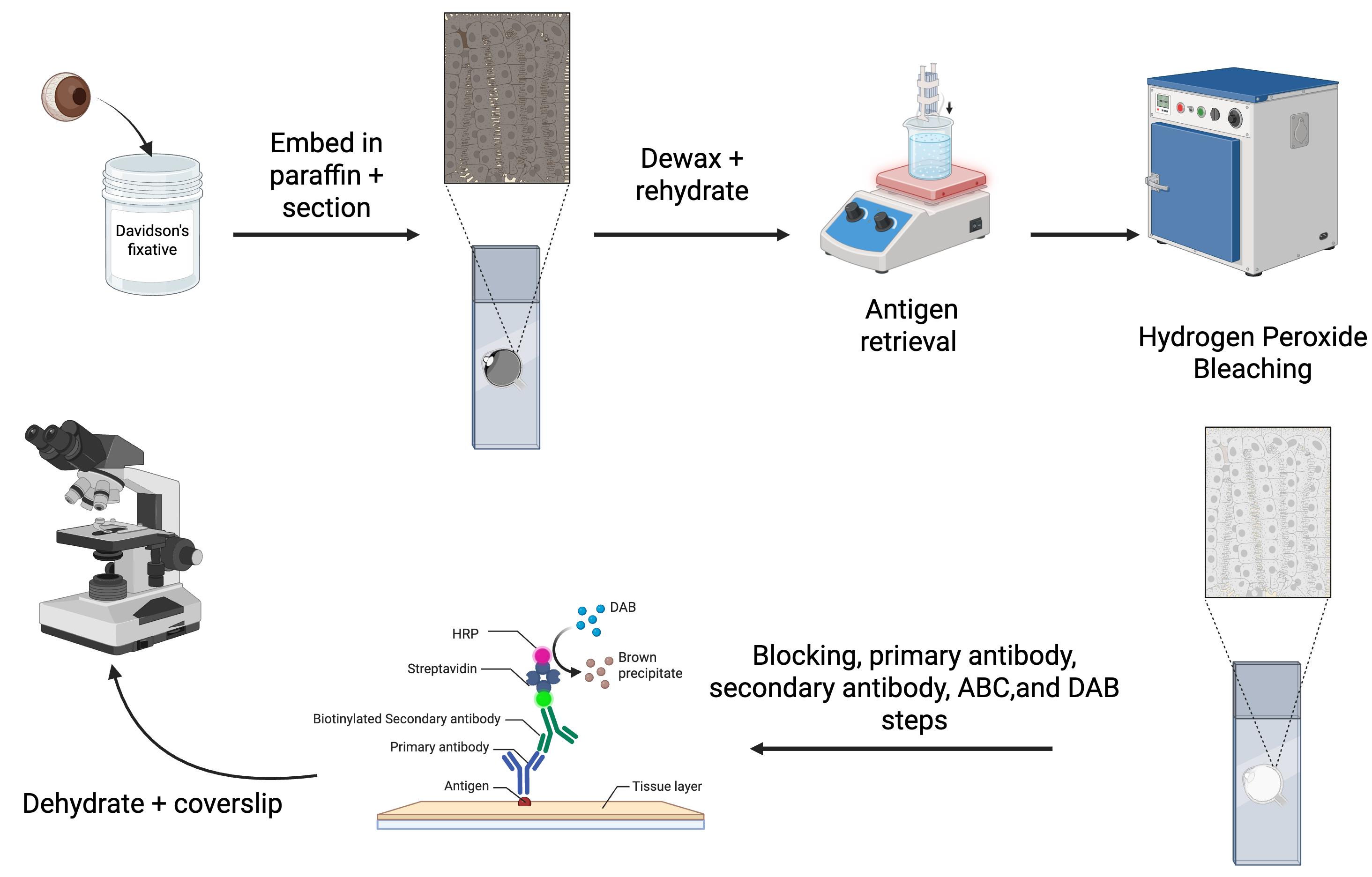
Overview of the immunohistochemistry protocol for eyes
Background
Immunohistochemistry (IHC) and immunofluorescence (IF) are essential techniques in molecular biology for assessing protein expression [1,2]. These methods, which rely on primary antibodies, can determine the presence and subcellular localization of proteins. This helps to identify processes such as cell division or activation of specific signaling pathways [2]. However, pigmentation in ocular tissues, particularly in the uveal tract (choroid, iris, ciliary body) and the retinal pigment epithelium (RPE), presents a significant challenge. Melanin interferes with both chromogenic and fluorescent detection methods, complicating the interpretation of IHC and IF results [3]. Additionally, distinguishing between the pigmented choroid and RPE, which are adjacent layers, can be difficult.
The most widely used chromogen for IHC is 3-3'-diaminobenzidine (DAB), which produces a brown precipitate that can be difficult to differentiate from melanin [3]. While azure B counterstaining can help distinguish between the DAB precipitate and melanin, its effectiveness is limited in highly pigmented tissues due to masking effects [3]. Alternatively, 3-amino-9-ethylcarbazole (AEC), a red chromogen, is an option, but its staining fades over time, making long-term storage of stained sections problematic [4]. Bleaching melanin before IHC has been explored, with methods such as potassium permanganate followed by oxalic acid, hydrogen peroxide, or trichloroisocyanuric acid (TCCA)[5]. However, these protocols have drawbacks, including reduced antigenicity and prolonged bleaching times, which can compromise tissue integrity [3].
In addition to pigment interference, the eye’s unique structure presents challenges in sectioning. Its hard crystalline lens and aqueous humor make it more difficult to slice than other tissues [1]. Formalin, the most commonly used fixative, can lead to cellular shrinkage, poor cellular detail preservation, and retinal detachment when used on ocular tissues [1]. Davidson’s fixative, a mixture of formaldehyde, ethanol, and acetic acid, is preferred for eye tissue IHC as it provides rapid tissue penetration and prevents excessive shrinkage [6].
In our lab, we study mouse models of uveal melanoma, which are highly pigmented tumors [7–13]. To overcome the challenges associated with pigment interference, we optimized an IHC protocol for mouse ocular tissues (Graphical overview). While not all primary antibodies are compatible with this method, we routinely use an S100b antibody to label melanocytes in the uveal tract and an RPE65 antibody for the RPE (Figure 1). In addition, we use a Ki67 antibody for uveal melanoma samples to label cells undergoing mitosis, and a CD68 antibody to identify melanophages. Melanophages are macrophages that have ingested melanin (Figure 2). In this paper, we present our optimized IHC protocol, which combines Davidson’s fixative with a hydrogen peroxide bleaching step to effectively eliminate melanin interference and improve protein detection in ocular tissues and uveal melanoma.
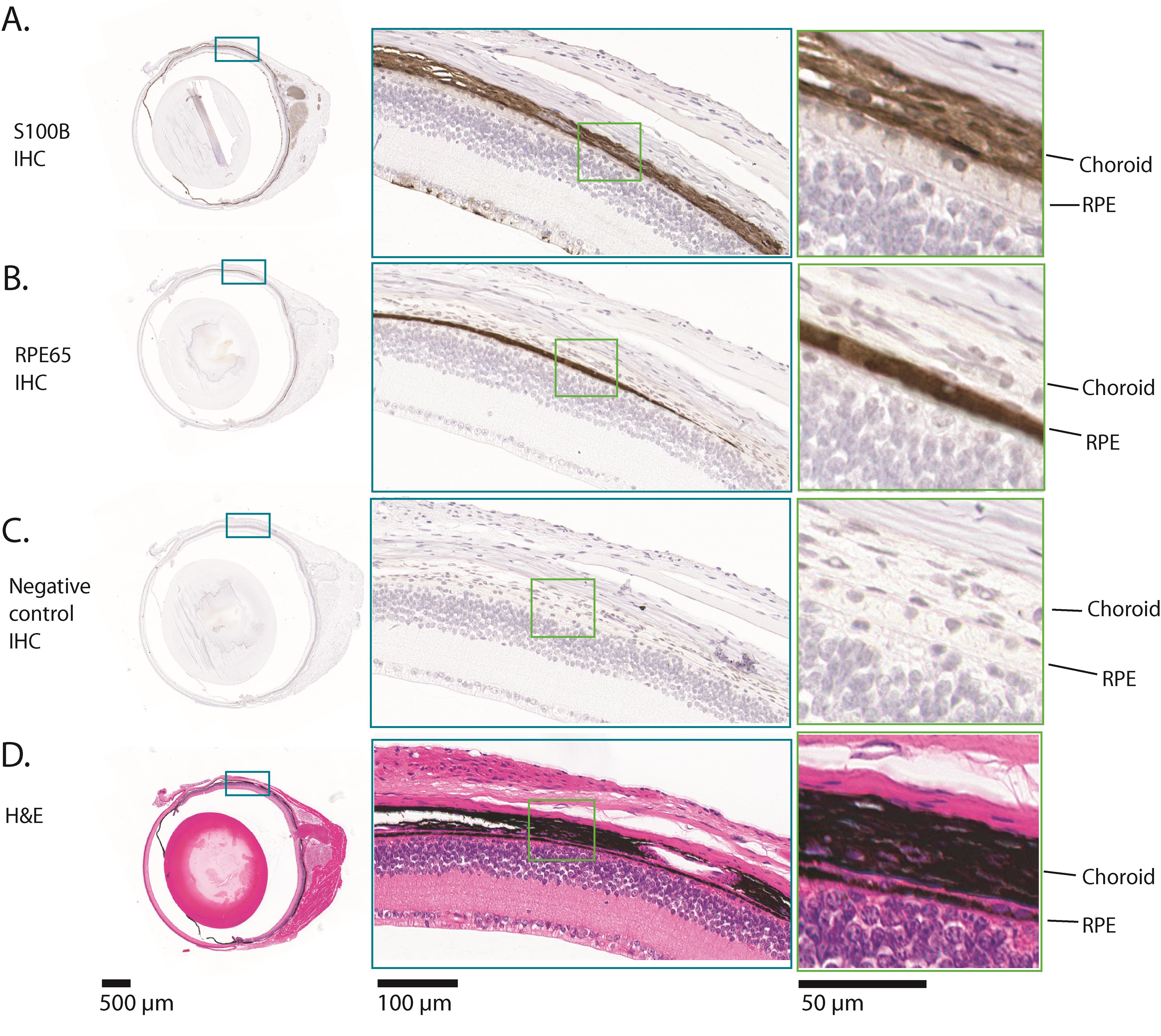
Figure 1. Example results of immunohistochemistry (IHC) for S100b and RPE65 on a normal adult mouse eye. The images shown are all serial sections from the same adult mouse eyeball. (A) This row shows the results of IHC for S100b. Melanocytes in the pigmented tissues, the choroid, ciliary body, and iris, express S100b, along with some cells in the innermost part of the neural retina and the optic nerve. The part of the eye that is enlarged shows S100b expression in the choroid. (B) This row shows the results of IHC for RPE65. RPE65 is expressed exclusively in the retinal pigment epithelium (RPE). The RPE is a monolayer of cuboidal cells. The part of the eye that is enlarged shows RPE65 expression. (C) This row shows the results of the negative control IHC, where no primary antibody was incubated with the section. There is no positive stain in any part of the eye. (D) This row shows a hematoxylin and eosin (H&E) stained section, in which there is abundant melanin in the choroid, iris, ciliary body, and RPE. Comparison of (D) with (C) shows that all melanin was removed during the hydrogen peroxide bleaching step. In each row, the teal and green boxes show progressively higher magnifications, from left to right.
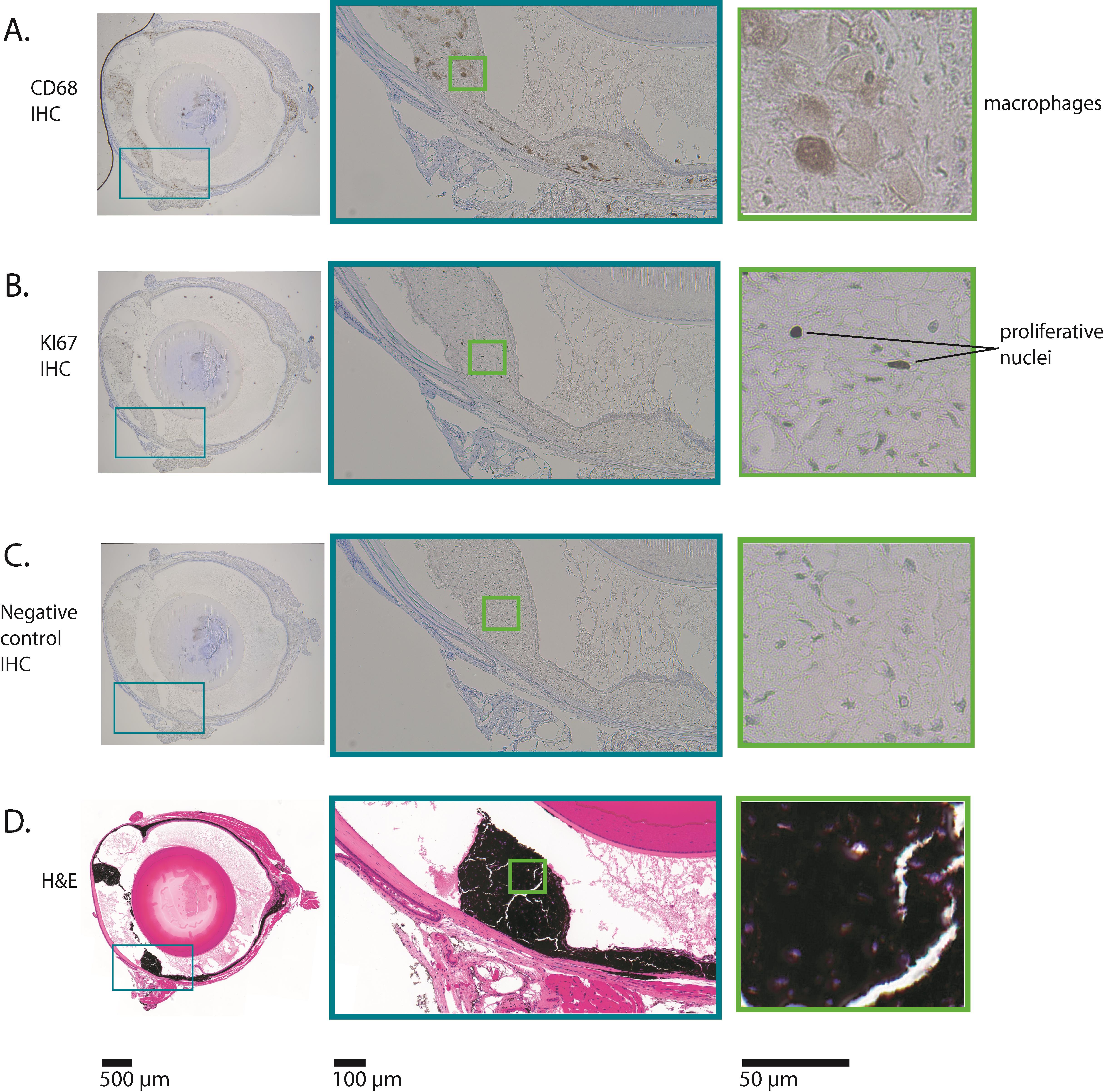
Figure 2. Example results of immunohistochemistry (IHC) for CD68 and Ki67 on a mouse eye with uveal melanoma. The images shown are all serial sections from the same adult mouse eyeball. This mouse expressed oncogenic GNAQQ209L via Mitf-cre (in melanocytes), which induced uveal melanoma. (A) This row shows the results of IHC for CD68. CD68 is expressed in macrophages. In this mouse uveal melanoma model, macrophages ingest melanin, becoming pigmented themselves. The macrophages in this section have abundant cytoplasm, consistent with active phagocytosis. The boxed area shows the part of the eye where the ciliary body and iris would have been located, but is now tumor tissue. (B) This row shows the results of IHC for Ki67. Ki67 is only expressed in nuclei that are undergoing mitosis. A small percentage of nuclei in this tumor are positive for Ki67 (indicated as "proliferative nuclei"), consistent with relatively slow growth. (C) This row shows the results of the negative control IHC, where no primary antibody was incubated with the section. There is no positive stain in any part of the eye. (D) This row shows a hematoxylin and eosin (H&E) stained section, in which there are very high levels of melanin in the thickened and abnormal iris, ciliary body, and choroid. Comparison of (D) with (C) shows that all melanin was removed during the hydrogen peroxide bleaching step. In each row, the teal and green boxes show progressively higher magnifications, from left to right.
Materials and reagents
Biological materials
1. Euthanized mouse
Note: This protocol has been successfully tested on a range of mouse ages, from newborn to adult.
Reagents
1. Paraffin (Fisherbrand, Histoplast PE, catalog number: 22900700)
2. VECTASTAIN® Elite® ABC-HRP Kit, peroxidase (rabbit IgG) (Vector Laboratories VECTPK6101), for use with anti-S100b, anti-CD68, and anti-Ki67 primary antibodies; this kit includes normal goat serum
3. VECTASTAIN® Elite® ABC-HRP Kit, peroxidase (mouse IgG) (Vector Laboratories VECTPK6102), for use with anti-RPE65 primary antibody; this kit includes normal horse serum
4. DAB Substrate kit peroxidase (with nickel) (Vector Laboratories, catalog number: SK4100)
5. Xylene histological grade (Fisher Chemical, catalog number: X3P-1GAL)
6. Hydrogen peroxide (H2O2) certified ACS 30% (Fisher Chemical, catalog number: H325-500), store at 4 °C
7. Triton X-100 (Sigma-Aldrich, catalog number: T8787-100ML)
8. Tween 20 (Sigma-Aldrich, catalog number: P9416-100ML)
9. Eukitt quick hardening mounting medium for microscopy (Sigma-Aldrich, catalog number: 03989-100ML)
10. Antigen unmasking solution, citric acid-based (100× stock citrate buffer) (Vector Laboratories, catalog number: H-3300)
11. Glacial acetic acid (Fisher Chemical, catalog number: A38-500)
12. Formaldehyde, 37% (Fisher Scientific, catalog number: BP531)
13. Distilled water
14. Ultrapure water (Purelab Flex 2; Elga Veolia)
15. 10× PBS (Sigma-Aldrich, catalog number: PPB006-20PAK)
16. Neutral buffered formalin, 10% (Simport, catalog number: M961-40FW)
17. Anti-S100b antibody (rabbit monoclonal antibody) (Abcam, catalog number: ab52642)
18. Anti-RPE65 antibody (mouse monoclonal antibody 401.8B11.3D9) (Thermo Fisher Scientific, catalog number: MA1-16578)
19. Anti-CD68 antibody (rabbit polyclonal antibody) (Abcam, catalog number: ab125212)
20. Anti-Ki67 antibody (rabbit polyclonal antibody) (Abcam, catalog number: ab15580)
21. Hematoxylin (Vector Laboratories, catalog number: H3401-500)
22. Ammonium hydroxide, ACS grade 30% (VWR, catalog number: BDH3014)
23. Hematoxylin (Vector Laboratories, catalog number: H3401-500)
Solutions
1. Davidson’s fixative (see Recipes)
2. Hydrogen peroxide bleaching solution (see Recipes)
3. 1× PBS/0.1% Tween 20 (see Recipes)
4. 1× PBS/0.3% Triton X-100 (see Recipes)
5. Blocking solution (see Recipes)
6. Primary antibody mix (anti-S100B or anti-KI67) (see Recipes)
7. Primary antibody mix (anti-RPE65) (see Recipes)
8. Primary antibody mix (anti-CD68) (see Recipes)
9. Secondary antibody mix (for S100b, CD68 and Ki67 assays) (see Recipes)
10. Secondary antibody mix (for RPE65 assays) (see Recipes)
11. ABC solution (see Recipes)
12. DAB-nickel solution (see Recipes)
13. Acid rinse solution (see Recipes)
14. Bluing solution (see Recipes)
Recipes
1. Davidson’s fixative
| Reagent | Final concentration | Volume (100 mL total) |
| Formaldehyde (37%) | 0.74% | 2 mL |
| Ethanol (100%) | 35% | 35 mL |
| Glacial acetic acid (100%) | 10% | 10 mL |
| Distilled water | n/a | 53 mL |
Note: Davidson’s fixative can be prepared ahead of time and stored in a glass bottle at room temperature.
2. Hydrogen peroxide bleaching solution
| Reagent | Final concentration | Volume (200 mL total) |
| 10× PBS | 1× | 20 mL |
| H2O2 (30%) at 4 °C | 10% | 66.6 mL |
| Ultrapure water | n/a | 113.4 mL |
3. 1× PBS/0.1% Tween 20
| Reagent | Final concentration | Volume (200 mL total) |
| 10× PBS | 1× | 20 mL |
| Tween 20 (100%) | 0.1% | 0.2 mL |
| Ultrapure water | n/a | 180 mL |
4. 1× PBS/0.3% Triton X-100
| Reagent | Final concentration | Volume (600 mL total) |
| 10× PBS | 1× | 60 mL |
| Triton X-100 (100%) | 0.3% | 1.8 mL |
| Ultrapure water | n/a | 538.2 mL |
5. Blocking solution
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal serum (100%) (goat serum for S100b, CD68, and Ki67 antibody assays, and horse serum for RPE65 antibody assay) | 5% | 2 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38 μL |
Note: Normal serum is included in the VECTASTAIN Elite ABC kits.
6. Primary antibody mix (anti-S100B or anti-Ki67)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal goat serum (100%) | 5% | 2 μL |
| Primary antibody | 1:1,000 dilution | 0.04 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38 μL |
7. Primary antibody mix (anti-RPE65)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal horse serum (100%) | 5% | 2 μL |
| Primary antibody | 1:250 dilution | 0.16 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38 μL |
8. Primary antibody mix (anti-CD68)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal goat serum (100%) | 5% | 2 μL |
| Primary antibody | 1:500 dilution | 0.08 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38 μL |
9. Secondary antibody mix (for S100b, CD68 and Ki67 assays)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal goat serum (100%) | 1.5% | 0.6 μL |
| Biotinylated goat anti-rabbit secondary antibody, from kit VECTPK6101 | 1:200 dilution | 0.2 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 39.2 μL |
10. Secondary antibody mix (for RPE65 assay)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal horse serum (100%) | 1.5% | 0.6 μL |
| Biotinylated horse anti-mouse secondary antibody, from kit VECTPK6102 | 1:200 dilution | 0.2 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 39.2 μL |
11. ABC solution
| Reagent | Final concentration | Volume (40 μL per eye section) |
| VECTASTAIN® Elite® ABC-HRP Kit Reagent A (use kit corresponding to primary antibody choice) | 2% | 0.8 μL |
| VECTASTAIN® Elite® ABC-HRP Kit reagent B (use kit corresponding to primary antibody choice) | 2% | 0.8 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38.4 μL |
12. DAB-nickel solution
| Reagent | Final concentration | Volume (400 μL per slide) |
| DAB Substrate kit, reagent 1 | 1.7% | 6.8 μL |
| DAB Substrate kit, reagent 2 | 2% | 8 μL |
| DAB Substrate kit, reagent 3 | 1.6% | 6.4 μL |
| DAB Substrate kit, reagent 4 | 1.6% | 6.4 μL |
| Ultrapure water | n/a | 372.4 μL |
13. Acid rinse solution
| Reagent | Final concentration | Volume (200 mL total) |
| Glacial acetic acid (100%) | 2% | 4 mL |
| Distilled water | n/a | 196 mL |
14. Bluing solution
| Reagent | Final concentration | Volume (200 mL total) |
| Ammonium hydroxide (30%) | 0.09% | 600 μL |
| Tap water | n/a | 200 mL |
Laboratory supplies
1. Disposable gloves (Fisher Scientific, catalog number: 191301597C)
2. Peel-A-WayTM disposable embedding molds (Fisher Scientific, catalog number: 2219)
3. Tissue PathTM disposable embedding rings (Fisherbrand, catalog number: 22-038197)
4. Thermal gloves (Sigma-Aldrich, catalog number: Z108286)
5. Pasteur pipette (Sigma-Aldrich, catalog number: Z627992)
6. Pasteur pipette rubber bulbs (Sigma-Aldrich, catalog number: Z111597)
7. Kimwipes (Sigma-Aldrich, catalog number: Z671584)
8. 4 × 1.5 mL Eppendorf tubes (Thermo Scientific, catalog number: 3448PK)
9. ImmEdge hydrophobic barrier PAP pen (Vector Laboratories, catalog number: H-4000)
10. 15 mL Falcon tube (Fisher Scientific, catalog number: 14-959-53A)
11. Parafilm (Sigma-Aldrich, catalog number: HS234526C)
12. 20 mL disposable glass scintillation vials (VWR, catalog number: 100500-676)
13. Superfrost Plus microscope slides (VWR, catalog number: 48311-703)
Equipment
1. Curved micro-dissecting forceps (Sigma-Aldrich, catalog number: F4142-1EA)
2. Shandon Histocentre 3 (Fisher Scientific, catalog number: 8358-30-1022)
3. 1,000 μL micropipette (Gilson, catalog number: F123602)
4. 200 μL micropipette (Gilson, catalog number: F123601)
5. 20 μL micropipette (Gilson, catalog number: F123600)
6. Microwave
7. Wash-N-Dry slide rack (Electron Microscopy Sciences, VWR, catalog number: 102096-892)
8. Hot plate (Corning, catalog number: 679540)
9. Precision compact oven (Thermo Scientific, catalog number: PR305225M)
10. Thermometer (Fisherbrand, catalog number: 13201521)
11. 1,000 mL glass beaker (Fisherbrand, catalog number: FB1001000)
12. Humidity/slide moisture chamber (Fisher Scientific, catalog number: 50-192-6622)
13. Glass slide tray (WHEATON®, catalog number: 900304)
14. 23 × Glass jars with lids (WHEATON®, catalog number: 900200)
15. Water bath (Thermo Scientific, model: Precision 2825)
16. Microtome (Leica, model: RM2255)
Procedure
文章信息
稿件历史记录
提交日期: Jun 23, 2025
接收日期: Oct 9, 2025
在线发布日期: Oct 23, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Longakit, A. N., Hess, C., Zhang, C. and Van Raamsdonk, C. D. (2025). Improved Immunohistochemistry of Mouse Eye Sections Using Davidson's Fixative and Melanin Bleaching. Bio-protocol 15(22): e5510. DOI: 10.21769/BioProtoc.5510.
分类
癌症生物学 > 通用技术 > 细胞生物学试验
细胞生物学 > 细胞染色 > 全细胞
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link