- EN - English
- CN - 中文
Characterizing Tissue Oxygen Tension During Neurogenesis in Human Cerebral Organoids
人脑类器官神经发生过程中组织氧张力的表征
(*contributed equally to this work, § Technical contact) 发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5507 浏览次数: 1703
评审: Sébastien GillotinAbhishek VatsSreesankar Easwaran

相关实验方案
基于图像的活细胞表型分析:Live Cell Painting 方法
Thaís Moraes-Lacerda [...] Marcelo Bispo De Jesus
2025年10月05日 1784 阅读
Abstract
Oxygen tension is a key regulator of early human neurogenesis; however, quantifying intra-tissue O2 in 3D models for an extended period remains difficult. Existing approaches, such as needle-type fiber microsensors and intensity-based oxygen probes or time-domain lifetime imaging, either perturb the organoids or require high excitation doses that limit the measurement period. Here, we present a step-by-step protocol to measure intra-organoid oxygen in human cerebral organoids (hCOs) using embedded ruthenium-based CPOx microbeads and widefield frequency-domain fluorescence lifetime imaging microscopy (FD-FLIM). The workflow covers dorsal/ventral cerebral organoid patterning, organoid fusion at day 12 with co-embedded CPOx beads, standardized FD-FLIM acquisition (470-nm external modulation, 16 phases at 50 kHz, dual-tap camera), automated bead detection and lifetime extraction in MATLAB, and session-matched Stern–Volmer calibration with Ru(dpp)3(ClO4)2 to convert lifetimes to oxygen concentration. The protocol outputs per-bead oxygen maps and longitudinal patterns stratified by bead location (intra-organoid vs. gel) and sample state (healthy vs. abnormal), enabling direct linkage between developmental growth and oxygen dynamics.
Key features
• End-to-end workflow linking hCOs generation, on-gel bead embedding, and FD-FLIM oxygen readout.
• Longitudinal single-organoid tracking of oxygen tension with bead-level metadata.
• Reference-based lifetime calibration and reproducible camera/LED settings.
• Ready-to-reuse materials, recipes, timing, and analysis logic.
Keywords: Human cerebral organoid (人脑类器官)Graphical overview
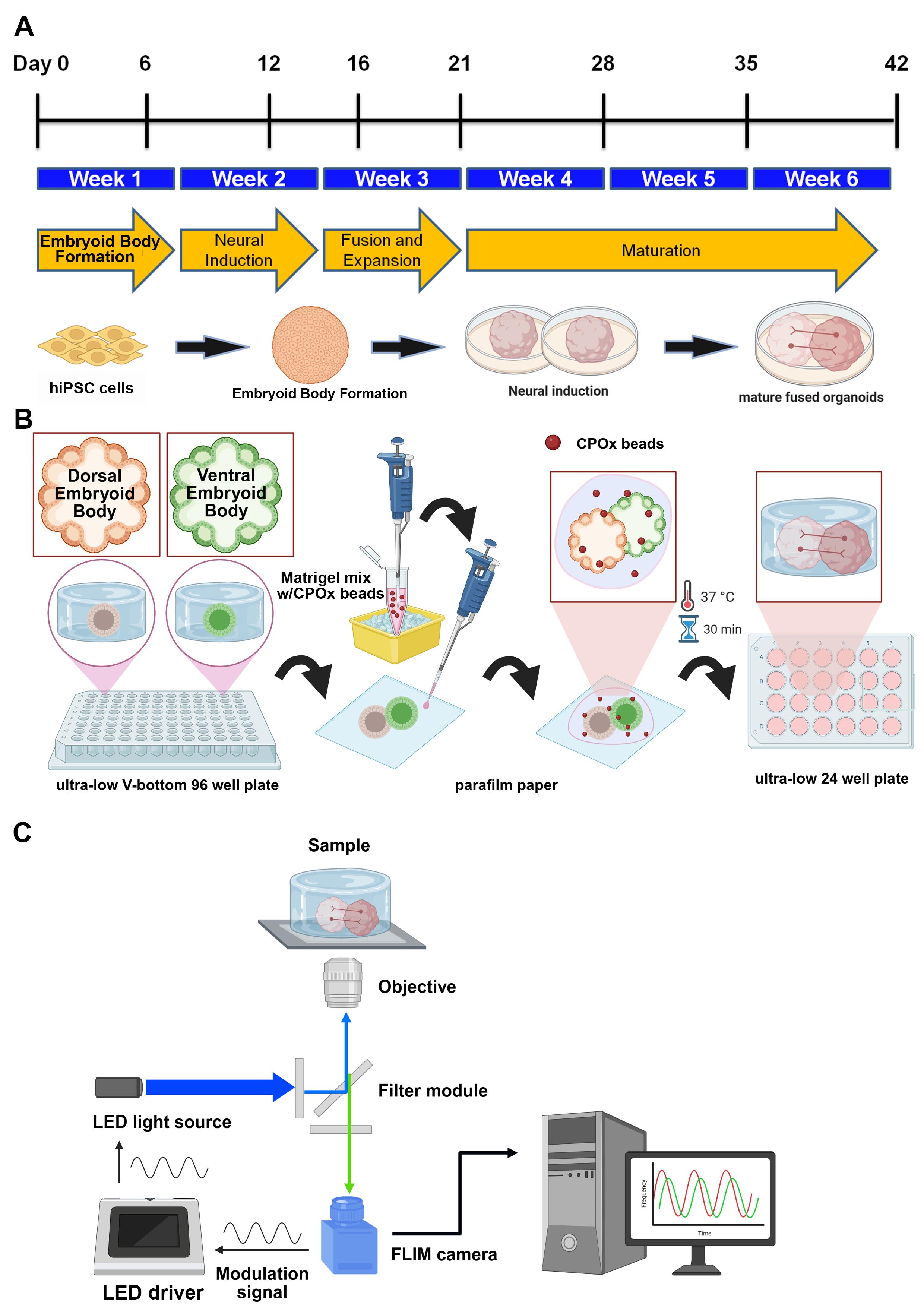
Workflow and timeline for fused human cerebral organoids (hCOs) generation. (A) Experimental timeline of dorsal/ventral embryoid bodies (EBs) patterning from human induced pluripotent stem cells (hiPSCs). Cerebral organoids were fused at day 12 and cultured for up to six weeks. (B) Schematic illustration for the incorporation of CPOx microbeads (oxygen sensor) during organoids fusion. (C) Schematic illustration for oxygen tension measurement through frequency-domain fluorescence lifetime microscopy (FD-FLIM).
Background
Oxygen availability is a central regulator of neural development, shaping stem/progenitor proliferation, neuronal differentiation, and metabolic state in the embryonic cortex [1–4]. Direct, longitudinal measurement of tissue oxygen in the developing human brain has been technically prohibitive. Human cerebral organoids (hCOs) offer an accessible, human-relevant platform to interrogate oxygen dynamics over time [5,6] and have been widely applied across neurological disease modeling contexts [6–8].
Approaches to monitor intra-organoid oxygen include needle-type optical fiber microsensors [6], which provide spatial oxygen profiles but are disruptive and unsuitable for repeated measurements over weeks. Non-disruptive, lifetime-based optical methods using oxygen-sensitive probes with phosphorescence lifetime imaging microscopy (PLIM)/fluorescence lifetime imaging microscopy (FLIM) mitigate intensity and ambient-noise confounds [9,10]. However, conventional time-domain lifetime implementations often require high excitation doses that risk photocytotoxicity in long-term live imaging, which makes it disadvantageous for live organoid observation over an extended period. In contrast, widefield frequency-domain (FD-FLIM) enables rapid, low-dose lifetime acquisition, making it suitable for longitudinal tracking of embedded oxygen sensors [11,12].
Building on this framework, our recent study used ruthenium-based oxygen-sensitive microbeads with FD-FLIM to identify a discrete developmental window (weeks 4–6) of markedly elevated intra-organoid oxygen tension that coincides with rapid neurogenesis and shifts in energy homeostasis [5]. Hypoxia or genetic perturbation of neuroglobin (NGB) attenuates this oxygen elevation and impairs neurogenesis, establishing a functional link between oxygen tension, metabolic programs, and developmental outcomes in hCOs. This protocol operationalizes that framework—detailing (i) hCO generation, (ii) bead co-embedding at fusion, and (iii) FD-FLIM configuration, acquisition, and analysis—so that other laboratories can reproduce longitudinal intra-organoid oxygen measurements and apply them to questions in human neurodevelopment, genetic perturbation (e.g., NGB), disease modeling, and microenvironmental manipulation.
Materials and reagents
Biological materials
1. Human induced pluripotent stem cells (hiPSCs) (RIKEN Bioresource Center, Cell No. HPS0076, clone 409B2)
Reagents
1. StemflexTM (Thermo Fisher Scientific, catalog number: A3349401)
2. Matrigel (hESC-qualified; coating) (Corning, catalog number: 354277)
3. Accumax (Sigma-Aldrich, catalog number: A7089)
4. AggreWellTM embryoid body (EB) formation medium (STEMCELL Technologies, catalog number: 05893)
5. DMEM/F12 (Thermo Fisher Scientific, catalog number: 12660012)
6. Neurobasal (Thermo Fisher Scientific, catalog number: 21103049)
7. N2 supplement (Thermo Fisher Scientific, catalog number: 17502048)
8. B27 supplement (without vitamin A) (Thermo Fisher Scientific, catalog number: 12587010)
9. B27 supplement (with vitamin A) (Thermo Fisher Scientific, catalog number: 17504044)
10. GlutaMAX (Thermo Fisher Scientific, catalog number: 35050061)
11. MEM-NEAA (Thermo Fisher Scientific, catalog number: 11140050)
12. Heparin (Sigma-Aldrich, catalog number: H0878)
13. ROCK inhibitor Y-27632 (Sigma-Aldrich, catalog number: SCM075)
14. Cyclopamine A (Sigma-Aldrich, catalog number: 239803)
15. IWP-2 (Sigma-Aldrich, catalog number: I0536)
16. SAG (Sigma-Aldrich, catalog number: 566660)
17. 2-Mercaptoethanol (Thermo Fisher Scientific, catalog number: 21985023)
18. Insulin (Sigma-Aldrich, catalog number: I9278)
19. DPBS (Ca2+/Mg2+-free) (Thermo Fisher Scientific, catalog number: 14190144)
20. Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9418)
21. CellMaskTM plasma membrane stains (Thermo Fisher Scientific, catalog number: C10046)
22. Ruthenium-based phosphorescent microbeads (CPOx beads) (Colibri Photonics, catalog number: CPOx-50-RuP)
23. Tris(4,7-diphenyl-1,10-phenanthroline)ruthenium(II) bis(perchlorate) [Ru(dpp)3(ClO4)2] (Toronto Research Chemicals, catalog number: TRC-T884520-500mg)
Solutions
1. Aggregation medium (see Recipes)
2. Neural Induction (NI) medium for dorsal patterning (see Recipes)
3. Neural Induction (NI) medium for ventral patterning (see Recipes)
4. CPOx beads stock solution (see Recipes)
5. CPOx-containing Matrigel solution (see Recipes)
6. hCOs expansion medium (see Recipes)
7. hCOs maturation medium (see Recipes)
Recipes
Note: The basal medium can be stored at -20 °C for six months and thawed immediately before use. However, as presented in the following recipes, all culture media can be stored for up to two weeks at 4 °C. The small molecules Y-27632, Cyclopamine A, IWP-2, and SAG should be added right before use. CPOx-containing Matrigel solution should be manipulated at 4 °C to prevent premature solidification of Matrigel.
1. Aggregation medium
| Reagent | Final concentration | Quantity or volume |
| AggreWellTM EB formation medium | 10 mL | |
| ROCK inhibitor Y-27632 (10 mM) | 10 μM | 10 μL |
2. Neural induction (NI) medium for dorsal patterning
| Reagent | Final concentration | Quantity or volume |
| DMEM/F12 medium | 10 mL | |
| GlutaMAX (100×) | 1:100 | 100 μL |
| MEM-NEAA (100×) | 1:100 | 100 μL |
| Heparin (1 mg/mL) | 1 μg/mL | 10 μL |
| N2 (100×) | 1:100 | 100 μL |
| Cyclopamine A (5 mM) | 5 μM | 10 μL |
3. Neural induction (NI) medium for ventral patterning
| Reagent | Final concentration | Quantity or volume |
| DMEM/F12 medium | 10 mL | |
| GlutaMAX | 1:100 | 100 μL |
| MEM-NEAA (100×) | 1:100 | 100 μL |
| Heparin (1 mg/mL) | 1 μg/mL | 10 μL |
| N2 (100×) | 1:100 | 100 μL |
| IWP-2 (5 mM) | 2.5 μM | 5 μL |
| SAG (10 mM) | 100 nM | 1 μL |
4. CPOx beads stock solution
| Reagent | Final concentration | Quantity or volume |
| DPBS | 1 mL | |
| CPOx (10 mg) | 10 mg/mL | |
| BSA (100%) | 1% | 10 μL |
5. CPOx-containing Matrigel solution
| Reagent | Final concentration | Quantity or volume |
| Matrigel | 100 μL | |
| CPOx beads stock solution (10 mg/mL) | 500 μg/mL | 5 μL |
6. hCOs expansion medium
| Reagent | Final concentration | Quantity or volume |
| DMEM/F12 medium | 25 mL | |
| Neurobasal | 25 mL | |
| GlutaMAX (100×) | 1:100 | 500 μL |
| MEM-NEAA (100×) | 1:200 | 250 μL |
| 2-mercaptoethanol (55 mM) | 19.25 μM | 17.5 μL |
| Insulin (10 mg/mL) | 2.5 μg/mL | 12.5 μL |
| N2 (100×) | 1:200 | 250 μL |
| B27 without vitamin A (50×) | 1:100 | 500 μL |
7. hCOs maturation medium
| Reagent | Final concentration | Quantity or volume |
| DMEM/F12 medium | 25 mL | |
| Neurobasal | 25 mL | |
| GlutaMAX (100×) | 1:100 | 500 μL |
| MEM-NEAA (100×) | 1:200 | 250 μL |
| 2-mercaptoethanol (55 mM) | 19.25 μM | 17.5 μL |
| Insulin (10 mg/mL) | 2.5 μg/mL | 12.5 μL |
| N2 (100×) | 1:200 | 250 μL |
| B27 with vitamin A (50×) | 1:100 | 500 μL |
Laboratory supplies
1. 6-well plates (Corning, catalog number: 3516)
2. Ultra-low-attachment 96-well round-bottom plates (Corning, catalog number: 7007)
3. Ultra-low-attachment 24-well plates (Corning, catalog number: 3473)
4. Microscope glass slide (Paul Marienfeld GmbH & Co. KG, catalog number: 1000000)
5. Microscope cover glass (diameter: 18 mm) (Paul Marienfeld GmbH & Co. KG, catalog number: 0111580)
Equipment
1. CO2-resistant shaker (Thermo Fisher Scientific, catalog number: 88881101B)
2. DMI6000 B inverted microscope (objective: 5×) (Leica Microsystems, catalog number: DMI6000B)
3. Hamamatsu ORCA-R2 cooled CCD camera (Hamamatsu Photonics K.K., catalog number: C10600-10B)
4. Dual-tap complementary metal-oxide semiconductor (CMOS) FLIM camera (Excelitas Technologies Corp., catalog number: PCO.FLIM)
5. High-power light-emitting diode (LED, nominal wavelength: 470 nm) (Thorlabs, catalog number: M470LP-C2)
6. LED driver (Thorlabs, catalog number: DC2200)
Software and datasets
1. Leica Application Suite X (LAS X) life science microscope software (Leica Microsystems, version: 3.4.2.18368)
2. Look@FLIM (version: 1.0.836-7, https://www.fish-n-chips.de/Look@FLIM/setup/, access date: 07/03/2025)
3. MATLAB (The MathWorks, Inc., version: 9.2.0.556344; R2017a)
4. ImageJ (U.S. National Institutes of Health)
5. SPSS (IBM Corp., version: 16)
6. Sigma plot (Grafiti, version: 14)
7. BioRender (https://app.biorender.com/)
8. AutoCAD (Autodesk Inc., version: 2020)
Procedure
文章信息
稿件历史记录
提交日期: Aug 31, 2025
接收日期: Oct 10, 2025
在线发布日期: Oct 23, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Liu, Y. H. and Wu, H. M. (2025). Characterizing Tissue Oxygen Tension During Neurogenesis in Human Cerebral Organoids. Bio-protocol 15(22): e5507. DOI: 10.21769/BioProtoc.5507.
- Liu, Y. H., Chung, M. T., Lin, H. C., Lee, T. A., Cheng, Y. J., Huang, C. C., Wu, H. M. and Tung, Y. C. (2025). Shaping early neural development by timed elevated tissue oxygen tension: Insights from multiomic analysis on human cerebral organoids. Sci Adv. 11(11): eado1164. https://doi.org/10.1126/sciadv.ado1164
分类
神经科学 > 发育 > 形态建成
细胞生物学 > 细胞成像 > 荧光
生物工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link