- EN - English
- CN - 中文
Immunopeptidomics Workflow for Isolation and LC-MS/MS Analysis of MHC Class I-Bound Peptides Under Hypoxic Conditions
缺氧条件下 MHC I 类分子结合肽的分离与 LC-MS/MS 分析免疫肽组学流程
发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5505 浏览次数: 1825
评审: Andrea GramaticaAnonymous reviewer(s)
Abstract
Immunopeptidomics enables the identification of peptides presented by major histocompatibility complex (MHC) molecules, offering insights into antigen presentation and immune recognition. Understanding these mechanisms in hypoxic conditions is crucial for deciphering immune responses within the tumor microenvironment. Current immunopeptidomics approaches do not capture hypoxia-induced changes in the repertoire of MHC-presented peptides. This protocol describes the isolation of MHC class I-bound peptides from in vitro hypoxia-treated cells, followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. It describes optimized steps for cell lysis, immunoaffinity purification, peptide elution, and MS-compatible preparation under controlled low-oxygen conditions. The method is compatible with various quantitative mass spectrometry approaches and can be adapted to different cell types. This workflow provides a reliable and reproducible approach to studying antigen presentation under hypoxic conditions, thereby enhancing physiological relevance and facilitating deeper immunological insights.
Key features
• Enables isolation of MHC class I-bound peptides from cells cultured under hypoxic conditions.
• Designed for low-input samples and optimized for maintaining cell viability during extended hypoxic exposure.
• Compatible with label-free LC-MS/MS for detailed immunopeptidome analysis.
• Adaptable to all human and murine cell lines commonly used in cancer and immunology research.
Keywords: MHC class I (MHC I 类分子)Graphical overview
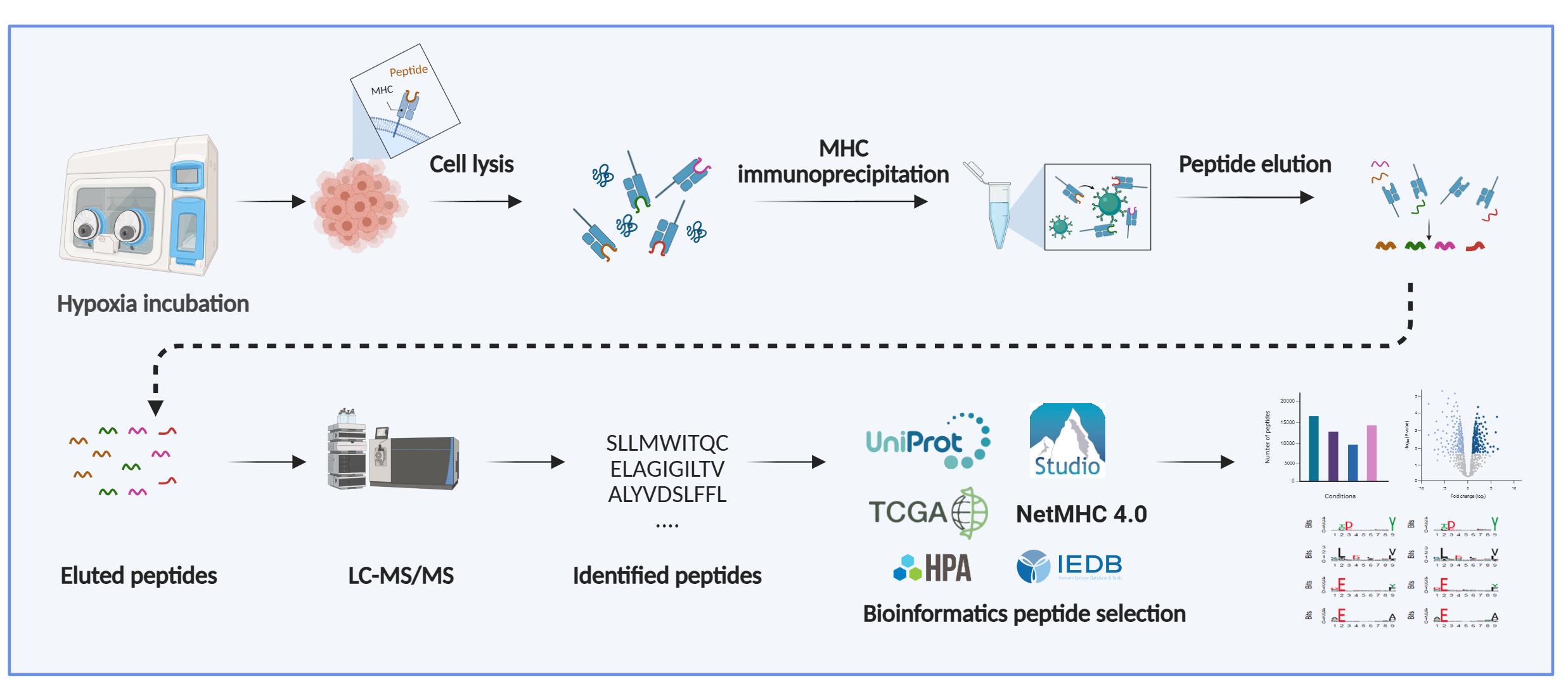
Background
The presentation of intracellular peptides by major histocompatibility complex (MHC) class-I molecules plays a central role in immune surveillance and the activation of cytotoxic CD8+ T lymphocytes [1–3]. Endogenous proteins are continuously degraded in the cytosol into peptide fragments, which are then transported into the endoplasmic reticulum (ER) by the Transporter Associated Protein (TAP) with antigen processing machinery. In the ER, peptides are trimmed to 8-11 amino acids and loaded onto MHC class-I molecules. These peptide-MHC class-I complexes are then transported to the cell surface, where they are recognized by CD8+ T cells for signs of infection, malignancy, or other cellular abnormalities [4,5]. The repertoire of the presented peptides by a given group of cells is termed the immunopeptidome. Recognition of a non-self or altered-self peptide derived from proteins can trigger targeted elimination of the presenting cell, thus contributing to pathogen clearance and tumor surveillance [6].
The ability to accurately identify MHC class I-bound peptides is essential for characterizing T-cell immunity at the level of individual tumor-specific peptides for developing more effective immunotherapies, including vaccines [7–9]. Mass spectrometry-based immunopeptidomics is a powerful methodology for characterizing the cancer cell immunopeptidome, including the discovery of tumor-specific antigens. [7,10]. However, the analysis of naturally presented MHC class I-bound peptides remains particularly challenging due to their low abundance, lack of standardized proteolytic cleavage, and high sequence similarity driven by MHC-binding preferences [11–13]. Additional challenges include optimizing sample preparation for diverse cell types, preventing loss of low-abundance peptides, addressing mass spectrometry biases, and adapting bioinformatic pipelines to reliably identify non-tryptic peptides.
The protocol presented here provides a reproducible workflow designed to facilitate robust immunopeptidomics analysis. Recent advances in mass spectrometry sensitivity, immunoaffinity purification protocols, and bioinformatic pipelines have significantly improved the depth and resolution of immunopeptidome analyses [14]. Importantly, the immunopeptidome reflects dynamic changes in cellular states, including those induced by stress, inflammation, or metabolic shifts, making it a rich source of potential diagnostic and therapeutic targets [15].
This growing interest in antigen presentation has led to the development of protocols capable of better capturing the full repertoire of peptides displayed by MHC class I and II under various physiological and pathological conditions, including hypoxia, a common feature of the tumor microenvironment [16,17]. Mapping these context-dependent changes is critical for identifying tumor-specific antigens, understanding immune escape mechanisms, and improving the efficacy of T cell-based therapies [18–20].
Materials and reagents
Biological materials
Cancer cell lines are grown in culture using adequate media and maintained in humidified incubators at 37 °C with 5% CO2. Caution: Routine validation of cell line identity (STR profiling) and mycoplasma testing is strongly recommended.
Reagents
Critical: Use only LC-MS-grade water, solvents, and high-purity reagents in all procedures. All glassware must be thoroughly cleaned with LC-MS-grade solvents (e.g., methanol) and LC-MS-grade water before use. Both glassware and plasticware can release polymeric contaminants, plasticizers, or detergent residues that interfere with peptide detection in LC-MS/MS analyses. To minimize such contamination, employ ultraclean glassware and high-quality, low-extractable plasticware. We strongly recommend verifying all tubes and consumables for leachable compounds before use. Where indicated, prepare solutions fresh before each experiment and discard any remaining solutions after no more than one week of storage. Use glass bottles for solution storage to reduce the risk of polymeric contaminants that can accumulate over time.
1. Formic acid (LC-MS ultra, >98% purity) (Sigma-Aldrich, catalog number: 10596814)
2. Acetic acid (glacial; HPLC-grade) (Sigma-Aldrich, catalog number: 10365020)
3. Acetonitrile (ACN) (LC-MS-grade) (Thermo Fisher Scientific, catalog number: 1.00029.2500)
4. Water (LC-MS-grade) (Thermo Fisher Scientific, catalog number: 1.15333.2500)
5. Trifluoroacetic acid (TFA) (LC-MS-grade) [Sigma-Aldrich, catalog number: 10723857 (A116-50)]
Caution: TFA is corrosive; wear appropriate personal protective equipment (PPE) accordingly. Buffers should be prepared in a fume hood due to the risk of toxic emissions.
6. Dimethyl pimelimidate dihydrochloride (Sigma-Aldrich, catalog number: D8388-250MG)
7. Complete protease inhibitor (Roche, catalog number: 11836145001)
8. IGEPAL® CA 630 (Abcam, catalog number: ab285400)
9. 0.5 M EDTA, pH 8 (Sigma-Aldrich, catalog number: 46-034-CI)
10. Protein A resin (Abcam, catalog number: ab270308-200mL)
11. Protein G resin (Abcam, catalog number: ab270309-100mL)
Critical: Check the isotype of the monoclonal antibody (mAb) to be used, as protein G resin may be more appropriate (e.g., for the IgG1 isotype). Refer to Table 1 for detailed information on the most commonly used anti-human antibodies.
Table 1. Commonly used anti-human antibodies for immunoaffinity capture of MHC-peptide complexes
| Hybridoma | Specificity | Isotype | Sepharose | ATCC |
|---|---|---|---|---|
| W6/32 | HLA -A, -B, -C | IgG2a | A/G | HB-95 |
| MA2.1 | HLA-A2, -B17 | IgG1 | G | HB-54 |
| BB7.2 | HLA-A2, -Aw69 | IgG2b | A/G | HB-82 |
| GAP A3 | HLA-A3 | IgG2a | A/G | HB-122 |
| TM1 | HLA-A2, -A28, -Bw4, -B27, -B44, -Cw2 | IgM | A | HB-169 |
| ME1 | HLA-B7, -Bw22, -B27, -B14, -Bw46 | IgG1 | G | HB-119 |
| DT9 | HLA-C (HLA-E) | IgG2b | A/G | - |
| 3D12 | HLA-E | IgG1k | G | Available under a licensing agreement from Fred Hutchinson Cancer Center |
12. PBS (1×, sterile) (Sigma-Aldrich, catalog number: J61196.AP)
13. H3BO3 (boric acid), pH 5.1 (25 °C, 1.8 g/L) (Sigma-Aldrich, catalog number: B6768-500G)
14. Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541-500G)
15. Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: S8045-500G)
16. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 31434-1KG-M)
17. Tris, ultra-pure grade (Sigma-Aldrich, catalog number: T6066-1KG)
18. pan MHC I antibody clone W6/32 (produced in house, hybridoma cells HB-95)
19. Dulbecco’s modified Eagle medium (Gibco, catalog number: 10313021)
20. Fetal bovine serum (Sigma-Aldrich, catalog number: F7524-500ML)
21. Penicillin-streptomycin (Sigma-Aldrich, catalog number: 15070063)
22. 0.25% trypsin-EDTA (Sigma-Aldrich, catalog number: T3924)
23. Purified mouse anti-human GRP78 (BD Biosciences, catalog number: 610978)
24. Purified mouse and-human HIF-1α (BD Biosciences, catalog number: 610959)
25. Goat anti-mouse IgG (H+L) (Licor Biosciences, catalog number: IRDye800RD)
26. LICOR blocking buffer (Licor Biosciences, catalog number: 927-60003)
27. 10 well, 50 μL gels (4%–20%) (Bio-Rad, catalog number: 4561094)
28. 12 well, 20 μL gels (4%–20%) (Bio-Rad, catalog number: 4561095)
29. Turbo Transfer kit (Bio-Rad, catalog number: 1704270)
30. Western Blot protein ladder (Bio-Rad, catalog number: 1610374)
Solutions
1. Boric acid-KCl stock solution (see Recipes)
2. Borate wash buffer (see Recipes)
3. Dimethyl pimelimidate (DMP) cross-linker (see Recipes)
4. Termination buffer (see Recipes)
5. 2× Lysis buffer (see Recipes)
6. Wash buffer 1 (see Recipes)
7. Wash buffer 2 (see Recipes)
8. Wash buffer 3 (see Recipes)
9. Wash buffer 4 (see Recipes)
10. 10% (v/v) acetic acid (see Recipes)
11. 1 M NaOH (see Recipes)
12. 0.1 M NaOH (see Recipes)
13. 1 M Tris, pH 8.0 (see Recipes)
14. 20% ACN 0.1% TFA (see Recipes)
15. 80% ACN 0.1% TFA (see Recipes)
16. 50% ACN 0.1% TFA (see Recipes)
17. 1% ACN 0.1% TFA (see Recipes)
18. 0.1% TFA (see Recipes)
19. Mass spectrometry loading buffer (see Recipes)
Recipes
1. Boric acid-KCl stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Boric acid | 0.1 M | 0.62 g |
| KCl | 0.1 M | 0.75 g |
| LC-MS-grade water | - | 100 mL |
2. Borate wash buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaOH | 0.1 M | 4 mL |
| Boric acid-KCl | 0.1 M | 50 mL |
| LC-MS-grade water | - | 100 mL |
3. Dimethyl pimelimidate (DMP) cross-linker
Prepare 40 mM DMP by dissolving 250 mg of DMP in 8 mL of borate wash buffer. Adjust the pH to 9.0 with 1 M NaOH. Top up to 25 mL with borate wash buffer. Prepare this solution fresh, immediately before use.
Critical: DMP powder can be stored at -20 °C; however, prolonged storage or repeated freeze–thaw cycles may reduce its cross-linking efficiency. To avoid stability issues, we recommend using single-use 250 mg vials, which are sufficient to crosslink up to 20 mg of antibody to 2 mL of resin.
4. Termination buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris pH 8.0 | 0.2 M | 20 mL from 1 M Tris stock solution |
| LC-MS-grade water | - | 80 mL |
5. 2× Lysis buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris pH 8.0 | 100 mM | 10 mL from 1 M Tris stock |
| NaCl | 300 mM | 20 mL from 1.5 M NaCl stock |
| IGEPAL | 1% | 10 mL from 10% IGEPAL |
| LC-MS-grade water | - | 60 mL |
Critical: 2× lysis buffer can be stored at 4 °C for 1–2 weeks. For optimal reproducibility, mix thoroughly before use and prepare fresh buffer if longer storage is required.
Add the protease inhibitor cocktail before use; dissolve one tablet in 10 mL of lysis buffer. Add phosphatase inhibitor if required (PhosStop, Roche) before use (1 tablet/10 mL of lysis buffer). Keep the lysis buffer cold.
6. Wash buffer 1
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris pH 8.0 | 50 mM | 5 mL from 1 M Tris stock |
| NaCl | 150 mM | 10 mL from 1.5 M NaCl stock |
| EDTA | 5 mM | 1 mL from 0.5 M EDTA |
| LC-MS-grade water | - | 84 mL |
7. Wash buffer 2
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris pH 8.0 | 50 mM | 5 mL from 1 M Tris stock |
| NaCl | 150 mM | 10 mL from 1.5 M NaCl stock |
| LC-MS-grade water | - | 85 mL |
8. Wash buffer 3
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris pH 8.0 | 50 mM | 5 mL from 1 M Tris stock |
| NaCl | 450 mM | 30 mL from 1.5 M NaCl stock |
| LC-MS-grade water | - | 65 mL |
9. Wash buffer 4
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris pH 8.0 | 50 mM | 5 mL from 1 M Tris stock |
| LC-MS-grade water | - | 95 mL |
10. 10% (v/v) acetic acid
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glacial acetic acid | 10% | 25 mL |
| LC-MS-grade water | - | 225 mL |
11. 1 M NaOH
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaOH (Mr = 40 g/mol) | 1 M | 4 g |
| LC-MS-grade water | - | 100 mL |
12. 0.1 M NaOH
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaOH (Mr = 40 g/mol) | 0.1 M | 0.4 g |
| LC-MS-grade water | - | 100 mL |
13. 1 M Tris, pH 8.0
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris (Mr = 121.14 g/mol) | 1 M | 121.14 g |
| LC-MS-grade water | - | 800 mL |
Adjust pH with HCl; approximately 42 mL of HCl is needed to achieve pH 8.0.
14. 20% ACN 0.1% TFA
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetonitrile | 20% | 10 mL |
| TFA | 0.1% | 50 μL |
| LC-MS-grade water | - | 39.95 mL |
15. 80% ACN 0.1% TFA
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetonitrile | 80% | 40 mL |
| TFA | 0.1% | 50 μL |
| LC-MS-grade water | - | mL |
16. 50% ACN 0.1% TFA
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetonitrile | 50% | 25 mL |
| TFA | 0.1% | 50 μL |
| LC-MS-grade water | - | mL |
17. 1% ACN 0.1% TFA
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetonitrile | 1% | 500 μL |
| TFA | 0.1% | 50 μL |
| LC-MS-grade water | - | 49.45 mL |
18. 0.1% TFA
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| TFA | 0.1% | 50 μL |
| LC-MS-grade water | - | 49.45 mL |
19. Mass spectrometry loading buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetonitrile | 1% | 500 μL |
| Formic acid | 0.1% | 50 μL |
| LC-MS-grade water | - | 49.45 mL |
Laboratory supplies
1. Petri dish, Duroplan, borosilicate glass, diameter: 143 mm, height: 30 mm (VWR International, catalog number: 391-0860)
2. 150 mm TC dish, sterile, non-pyrogenic (Fisher Scientific, catalog number: 11804125)
3. TC cell scraper (Sarstedt, catalog number: 83.3951)
4. 10 μL filter pipette tips, hinged, extended length, graduated (Appleton Woods, catalog number: ACA619)
5. 20 μL filter pipette tips, hinged, extended length, graduated (Appleton Woods, catalog number: ACA626)
6. 200 μL filter pipette tips, hinged, extended length, graduated (Appleton Woods, catalog number: ACA659)
7. 1,250 μL filter pipette tips, hinged, extended length, graduated (Appleton Woods, catalog number: ACA668)
8. Protein LoBind tube 1.5 mL, PCR clean (Fisher Scientific, catalog number: 10708704)
9. Protein LoBind tube 2 mL, PCR clean (Fisher Scientific, catalog number: 10718874)
10. Econo-Column® chromatography columns (Bio-Rad, catalog number: 737-1012)
11. Ultrafree®-MC centrifugal filter units (Merck, catalog number: UFC3LCCNB-HMT)
12. Falcon 15 mL centrifuge tubes (Fisher Scientific, catalog number: 352097)
13. Falcon 50 mL centrifuge tubes (Fisher Scientific, catalog number: 352098)
14. PierceTM centrifuge columns (Thermo Fisher Scientific, catalog number: 89868)
15. PierceTM C18 spin tips, 96 tips (Thermo Fisher Scientific, catalog number: 84850)
16. Certified kits, screw thread vials, 12 × 32 mm, 9 mm thread, unassembled (Merck, catalog number: 29385-U)
17. pH strips (Fisher Scientific, catalog number: 10642751)
18. PierceTM BCA Protein Assay kit (Thermo Scientific, catalog number: 23225)
19. Anaerobic indicator strips (Fisher Scientific, catalog number: BR0055B)
Equipment
1. Calibrated pH meter
2. Benchtop centrifuge
3. Chromatography column (Bio-Rad, model: Econo-Column®)
4. Shaker (IKA, model: VX 2E.n)
5. Orbital rotator
6. Tube rotator (Camlab, catalog number: 51901-26)
7. Ultrasonic USC-TH sonicator bath
8. Hypoxia chamber (Don Whitley Scientific)
9. Racks (Thermo Fischer)
10. Freezer (-20 °C and -80 °C)
11. Refrigerator (2–8 °C)
12. LC-Mass Spectrometer
13. Trans-Blot Turbo system (Bio-Rad)
14. Buffer tank and lid (Bio-Rad)
15. Gel holder cassette (Bio-Rad)
16. OxyLite probe (Oxford Optronix)
Software and datasets
High-resolution mass spectrometry generates large datasets that require computational processing to identify peptide sequences from fragmentation spectra. Common search algorithms, including MASCOT, SEQUEST, MaxQuant, and Protein Pilot, perform this task by searching spectra against fragmentation spectra databases and de novo peptide sequencing. In immunopeptidomics, where MHC-bound peptides are not produced by enzymatic digestion and therefore do not follow trypsin-specific cleavage rules [cleavage after arginine (R) and lysine (K)], hybrid approaches are strongly recommended. Computational tools like PEAKS combine database searching with de novo sequencing, first generating candidate peptide sequences through de novo analysis to guide the database search. This strategy enhances both search efficiency and identification accuracy, which is critical given the non-tryptic nature of MHC-associated peptides. In this protocol, we used PEAKS Studio v10.0 (Bioinformatics Solutions Inc.), as its integration of de novo sequencing with database searching provides improved identification of non-tryptic peptides compared to conventional search engines focused on tryptic peptides.
Note: PEAKS (commercial license required; https://www.bioinfor.com/peaks/), NetMHC (freely available; https://services.healthtech.dtu.dk/services/NetMHCpan-4.1/), IEDB (freely available; https://www.iedb.org/), WebLogo (freely available; http://weblogo.threeplusone.com/), FragPipe (freely available; https://fragpipe.nesvilab.org/), and MHCquant 2 (freely available; https://www.mhquant.org/) are commonly used in these analyses.
Procedure
文章信息
稿件历史记录
提交日期: Aug 19, 2025
接收日期: Oct 8, 2025
在线发布日期: Oct 23, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Estephan, H., Hammond, E. M. and Adamopoulou, E. (2025). Immunopeptidomics Workflow for Isolation and LC-MS/MS Analysis of MHC Class I-Bound Peptides Under Hypoxic Conditions. Bio-protocol 15(22): e5505. DOI: 10.21769/BioProtoc.5505.
分类
癌症生物学 > 微环境 > 低氧
免疫学 > 免疫机理
生物化学 > 蛋白质 > 定量
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link